Nitrogen: The bringer of life and death
- Published

Nitrogen is one of the most paradoxical elements in the periodic table. Flames are extinguished and animals die in an atmosphere of pure nitrogen - so it was once known as "azote", Greek for "lifeless". And yet this colourless, odourless gas, making up 78% of the atmosphere, has a highly explosive nature.
For mankind nitrogen is the bringer of life - and death - on an epic scale.
Atmospheric nitrogen is N2, a molecule consisting of two nitrogen atoms held together with an incredibly strong "triple bond", in which the atoms pool six electrons. But split this bond apart and nitrogen's nature changes dramatically.
"The flip side to nitrogen's incredible stability is the fact that some of its compounds turn out to be very, very reactive," explains Prof Andrea Sella in his laboratory on the University College campus in central London.
He picks up a long wooden pole and uses it to point to a small mound of a greyish purple powder sitting on a board on one of the lab tables.
"Cover your ears," he warns. Then he taps the mound with the pole.
I'm expecting something dramatic, but I still yelp at the violence of the detonation. Sella laughs.
The bang rings in my ears and a mysterious whiff of purple smoke rises up from a scorch mark where the powder was.
"That is a very famous compound called nitrogen tri-iodide," he tells me with a grin.
"Like almost all explosives, it exploits the tendency of nitrogen to form that triple bond."
Nitroglycerin and TNT (Trinitrotoluene) are compounds of nitrogen, oxygen, hydrogen and carbon, while one of gunpowder's main constituents is potassium nitrate. Nitrogen tri-iodide, for its part, is made of nitrogen and the purple-coloured iodine.

These compounds are held together by relatively weak bonds. When they are broken, the nitrogen atoms form strong triple bonds with each other releasing huge amounts of energy.
What's more, Sella explains, you are taking a solid and converting it into a gas, so the substance expands by close to 1,000 times.
"This combination of expansion and heat really gives us incredible power," he says.
The history of explosives in warfare and mining provides eloquent evidence of that. But nitrogen also plays a crucial role in sustaining life.
DNA and RNA - the molecules which carry the instructions for life - include nitrogen. So do amino acids, the basic units that make up proteins, which Sella describes as the machines of the biological world.
"They provide the catalysts, the little engines that do all of the hard work inside cells," he says.
"They operate as pumps, moving molecules and ions around, they transform one molecule into another, they separate the DNA and do the details of the copying."
And because plants need nitrogen to build these little machines, nitrogen is an essential fertiliser.
But like explosives, plants can only use reactive nitrogen. The triple bonds that make atmospheric nitrogen a tough brick of a molecule must be broken - but how?
Certain bacteria, says Sella, are capable of performing an extraordinary chemical conjuring trick.
"They have learned to use metal atoms embedded in a matrix to attack nitrogen, bombarding it with a mixture of electrons and protons at the same time," he says.
This converts atmospheric nitrogen (N2) into ammonia (NH3) - which plants can use.
"The entire living world is based on this nitrogen fixation process," says Sella.
This has led to one of the most profitable symbiotic relationships in all nature.
Certain plants, notably "legumes" such as peas, beans and clover, nurture the bacteria in nodules attached to their roots. They secrete sugars to feed the bacteria and in return the bacteria supply the plants with a ready supply of nitrogen - and when the plants die, any remaining nitrogen is returned to the soil.
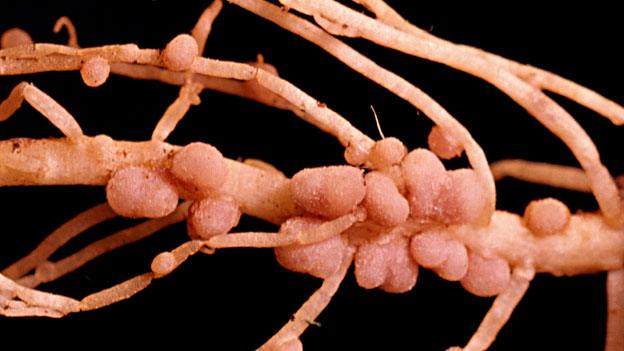
In short, such plants are excellent natural fertilisers, which is why they are known as "green manure".
Farmers spotted at least 8,000 years ago how useful legumes could be. Growing them in rotation with other crops like cereals helped kept the soil nitrogen-rich, and at the same time they produced nourishing high-protein seeds (think soya beans and chick peas).
While crop rotation continued to be perfected right up until the 18th Century (notably by the British aristocrat Viscount Charles "Turnip" Townshend) there is a limit to how much nitrogen can be extracted from the air and delivered to crops by this technique.
So with European and American populations growing rapidly in the 19th Century, the world was hungry for fertiliser - and in 1864 it experienced its first nitrogen war. Spain and Peru used nitrogen-based explosive weapons to secure access to the nitrogen resources of the craggy Chincha Islands in the Pacific Ocean.
The source of the element was none other than bird poo - guano - deposited by generations of sea birds over thousands of years.

Guano often gives islands a thick white crust
With help from Chile, Peru won the war. But a decade later, the guano was already running out, and so attention shifted to another source of nitrogen close at hand - the saltpetre flats of the Atacama desert.
And, naturally enough, it wasn't long until former allies Chile and Peru went to war with each other over the Atacama.
Which prompts a question.
The world's population has increased five-fold since the 1870s. That's a lot of mouths to feed. So where does all the nitrogen fertiliser come from today? Why are there no more nitrogen wars?
I travelled to Ludwigshafen in Germany, to find out.

This nondescript city is home to one of the most important scientific inventions in history - the Haber-Bosch process. It has been described as miraculous, creating "bread from air", and it was at the BASF chemical plant that the industrial process for fixing nitrogen from the air to make ammonia was developed.
The key breakthrough was made in 1909 by an ambitious young chemist called Fritz Haber, who conducted a table-top experiment to demonstrate that it was possible. But there was a problem - the process required almost 200 atmospheres of pressure and temperatures of over 400C (752F).

Haber (left) won a Nobel prize in 1918, Albert Einstein (right) won his in 1921
BASF engineers led by Carl Bosch overcame the challenge in a feat comparable to that of an Apollo Moon mission, says Dr Michael Mauss, who used to run the vast ammonia plant, one of the largest chemical facilities in the world.
"The financial risk to the company was tremendous," he tells me when we meet in a pretty park on the edge of the plant. "Everything had to be invented - the compressors, the catalysts and the reactors that could bear these huge temperatures and pressures."
But by 1912 BASF had got the plant working.
Mauss is adamant that the company's interest in nitrogen was the necessity to head off a looming food crisis - but to begin with the plant's output was used for a very different purpose. With World War One looming, the ammonia was diverted into munitions production. Once again, nitrogen's dual nature was apparent.
These days, explosives make up only a tiny part of the market for the huge quantities of ammonia produced using the Haber-Bosch process. Most goes to manufacture fertiliser, and all this "synthetic" nitrogen has vastly increased agricultural output across the world over the years.
The majority of the nitrogen in your body probably came from the Haber-Bosch process. And without it, more than half of the world's population would have nothing to eat.
The incredible yields synthetic fertilisers deliver explain why obesity has replaced hunger as the rich world's biggest nutritional challenge.
And there are other drawbacks.
All the extra nitrogen mankind is extracting from the air has to end up somewhere. Some of it passes through our bodies, and those of our animals, and is flushed away in sewage.
Still more is washed straight off the fields into rivers by heavy rain, or leaches into groundwater. All this causes ecosystems to become overloaded with nitrogen, a process known as "eutrophication". What happens is great blooms of algae, and then bacteria, feed on the surplus nitrogen. In the process, they suck all the oxygen from the water, killing fish and other organisms.

An algal bloom on Lake Atitlan in Guatemala
"By 2050, we're looking at getting huge amounts [of reactive nitrogen] into our oceans," says Giles Oldroyd, of the John Innes Centre, a century-old agricultural research centre in Norwich. "It has the potential to see a massive collapse of our oceanic systems, and all the fish that we are dependent on for our food."
He is trying to figure out how to transfer the complex set of genes responsible for creating those nodules in legumes such as peas, across to major cereal crops such as rice and wheat.
"I hope I can achieve it within my career, and I've got 30 years until I retire," he says.
If Oldroyd and his team are successful, it would massively reduce the world's dependence on the Haber-Bosch process. Not only would that reduce eutrophication, it would also cut greenhouse gas emissions - Haber-Bosch is reckoned to use 1% of the world's energy supplies, which reflects just how hard it is to rip those triple-bonds apart.
Dr Beth McGee on the threat nitrogen poses to Chesapeake Bay on the US Atlantic coast
But what became of that eager young chemist, Fritz Haber, whose table-top experiment first solved the world's nitrogen crisis?
A fervent German nationalist, he not only helped, as we have seen, supply Germany with explosives during World War One- he went on to develop chemical weapons.
This was too much for his wife, Clara, herself a chemist. Just after his new poison gases were first put to work in the trenches, she took his service revolver and shot herself. Fritz left the very next morning to oversee the gas's use on the Eastern Front.
The Nobel Prize judges weren't as critical of his wartime work as his wife. In 1918 he was awarded the Nobel Prize for Chemistry for his work on nitrogen. And after the war, he used his know-how to develop pesticides - including that notorious group of nitrogen-based toxins, the cyanides.
His work then came to an abrupt end in 1933.
Although he had converted to Lutheranism, Fritz Haber had been born Jewish, and as far as the Nazis were concerned he had no place in the new Reich. He fled to England, only to be rejected by his fellow chemists because of his wartime record.
A year later he headed for Palestine, but died of a heart attack en route. And perhaps it was for the best.
Had he lived, Fritz Haber would have seen most of his extended family in Germany wiped out by Zyklon B, a poison gas whose development he had overseen, and whose manufacture depended on the process of nitrogen fixation that he had pioneered.
Subscribe to the BBC News Magazine's email newsletter to get articles sent to your inbox.