Huntington's breakthrough 'amazing' but with 'caveats'

Dr Lauren Byrne said Wednesday's news of a successful Huntington's trial was "an amazing step"
- Published
A woman whose father died from Huntington's disease has said news that it has been successfully treated for the first time is "an amazing step", but cautioned there were "caveats" to the breakthrough.
Dr Lauren Byrne said her dad, who died earlier this year, was one of eight siblings "and so far five of them have it and there's now around 40 people at risk at my last count".
The disease runs through families, relentlessly kills brain cells and resembles a combination of dementia, Parkinson's and motor neurone disease.
She said: "You have to watch your loved one get sick for decades and look and see your own future if you are at risk."
Dr Byrne, who is originally from Newcastle in County Down, is a principal research fellow at University College London (UCL) Huntington's Disease Centre and a trustee on the Huntington's Disease Association Northern Ireland.
She was not directly involved in the trial, but has worked closely with the UCL team who made the breakthrough.
The UCL research team told the BBC the data shows the disease was slowed by 75% in patients.
Dr Byrne told the Good Morning Ulster programme, Huntington's was "such a debilitating disease that has such a wide impact for the whole family.
"I'm gene-negative, but the majority of my family are still at risk, including my siblings."

Lauren's dad Peter died earlier this year with Huntington's
Dr Byrne said "it was a crazy day in the office yesterday" as she watched the news about the successful trial come out with some of her colleagues.
"It's definitely a momentous result for the community," she said.
"As a scientist it's amazing, this is proof of concept that lowering Huntington's in the brain of Huntington's patients can slow disease symptoms and we can't take away from that.
"There's also caveats - it's a very small trial, around 30 participants," she explained.
"Its gene therapy administered through brain surgery, so there's high risks involved."

Dr Byrne is a principal research fellow at University College London (UCL) Huntington's Disease Centre
Dr Byrne said work will now be undertaken to get the new treatment approved in the US next year, but it will then take time to make it available in the UK and Europe.
As a result, she said she found herself "trying to balance the optimism".
However, she said the successful trial "has a further impact beyond this therapy itself".
"There's multiple different drugs that are in different stages of development that are also Huntington lowering – maybe a pill or an injection into the spine, so lots of different ways," she said.
"The fact that this works is going to have a real positive benefit for all of these programmes.
"So there's a lot of hope for the community at the minute. even if someone can't get this, there are other things coming that will be easier to get access to."

Peter Byrne was one of eight children, five of whom went on to have Huntington's disease
'Good quality life'
Prof Sarah Tabrizi, director of the University College London Huntington's Disease Centre, described the trial results as "spectacular".
She said they mean the decline you would normally expect in one year would take four years after treatment, giving patients decades of "good quality life",
The first symptoms of Huntington's disease tend to appear in your 30s or 40s and is normally fatal within two decades – opening the possibility that earlier treatment could prevent symptoms from ever emerging.
What is Huntington's disease?
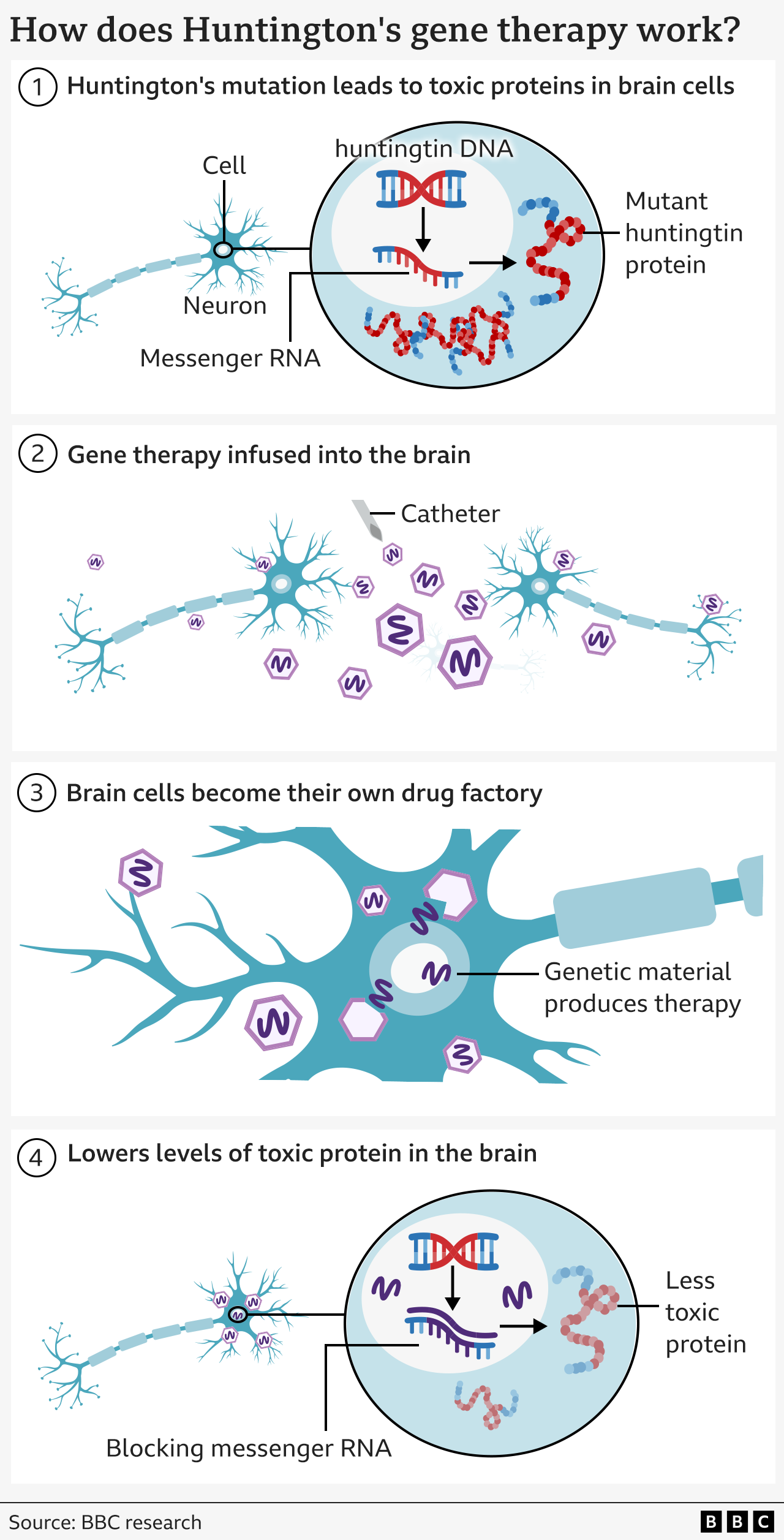
Huntington's disease is caused by an error in part of our DNA called the huntingtin gene.
If one of your parents has Huntington's disease, there's a 50% chance that you will inherit the altered gene and will eventually develop Huntington's too.
This mutation turns a normal protein needed in the brain – called the huntingtin protein – into a killer of neurons.
The goal of the treatment is to reduce levels of this toxic protein permanently, in a single dose.
The therapy uses cutting edge genetic medicine combining gene therapy and gene silencing technologies.
Related topics
- Published24 September
