Huntington's breakthrough 'like winning the lottery 10 times over'

Matt and Gemma Botting with their children Amelie and Hugo
- Published
Gemma Botting burst into tears when she saw the news headlines this week about a treatment for Huntington's.
"I must have cried for three hours. Then the kids got home from school and I showed my daughter the BBC story and she burst into tears."
Gemma's husband, Matt, was diagnosed with Huntington's disease in 2011. The condition resembles a combination of dementia, Parkinson's and motor neurone disease (MND) and is normally fatal within two decades after symptoms start to show. Matt became symptomatic two years ago.
Until now there has been no treatment. But on Wednesday researchers reported how a new gene therapy had managed to slow progress of the disease by 75%.
"It is like winning the lottery 10 times over," says Gemma, 45, who contacted Your Voice, Your BBC News after the breakthrough was announced. "I had accepted I would never get to grow old with my husband and that he might never see the kids become adults. That has all changed now."

Matt and Gemma on holiday in Egypt last year
Making the most of their time together
Gemma, who lives just outside Swindon with Matt and their two children, Amelie, 11, and Hugo, eight, says they have tried to make the most of the time they have had together.
When he was diagnosed, they took a break from their jobs in the logistics industry to travel the world. "We went for a year – got all our holidays in at once before we had the children," Gemma says.

Matt and Gemma got married on the way to Machu Picchu, Peru, during their year travelling the world
But even having children was something that took some navigating. They decided they wanted to screen out the gene that causes the disease. They had two options – either have a version of IVF whereby the embryo is tested for the gene before implantation, or conceive naturally and have the developing embryo tested.
They opted for the latter, but to be eligible for this couples have to agree to abort the foetus if the test is positive, as it was in their second pregnancy.
"We thought carefully about what we wanted to do. In the Huntington's community not everyone agrees with screening it out. But we both knew we did not want our children growing up with the possibility of having this gene. It is such a cruel, cruel disease."
'Hope where there was no hope'
Matt, now 43, is still able to walk, but has become clumsy with his movement and also been affected mentally.
"He has angry outbursts and has no empathy altogether. For example, if one of the children hurts themselves he will laugh. It's worse when he's tired. That's how Huntington's works – it alters your personality and that is incredibly hard for the children to understand."
Matt no longer works, having had to retire on health grounds when his symptoms started to develop.
"In some ways we are lucky he got past 40 with no signs of it," says Gemma. "His mother died from the disease when she was 40. And now we have the prospect of a treatment. We have got hope where there was no hope."
She says that is a sentiment that will be felt by the whole Huntington's disease community.
"I work in the evenings as a counsellor, often with people with Huntington's disease. I trained partly to give something back to the community and because we needed the money as Matt is no longer earning.
"I often support people just after they have been tested for it. Depression is the most common issue as they feel all is lost. That is why the news about the treatment is so important. It will give everyone a lift," she explains.
"Our neighbours and friends are even talking about fundraising so we can pay for the treatment. I just hope it is made available on the NHS quickly."
That, of course, is the question on the mind of everyone who has been affected by the disease.
The BBC has been contacted by numerous families who have lost whole generations to the illness and others who have lost their loved ones to suicide because they were unable to face the inevitable decline that follows a diagnosis.
Dave, 73, from the West Midlands, lost his wife to Huntington's. His son now has the disease and he is worried the gene may have been passed on to his granddaughter.
"It is horrible to see your loved ones suffer," he says. "This treatment is desperately needed on the NHS."
The path to the NHS
Whether and how quickly the treatment will become available is not yet clear.
The company behind the therapy, uniQure, says it will apply for a licence in the US in the first quarter of 2026.
Once regulators there have assessed it and, if they approve it for use, the UK regulator, the Medicines and Healthcare Regulatory Agency (MHRA), can piggyback off their work and put it through a quick regulatory process.
But that is only to decide if it is safe and effective. It will be up to another body, the National Institute for Health and Care Excellence (NICE), to decide if it is affordable to be used on the NHS in England and Wales. A separate body makes that calculation for Scotland.
As a gene therapy involving brain surgery, it will be expensive. However, sources at NICE say that is not necessarily a deal-breaker. They point to the fact that a sickle cell therapy that costs £1.65 million per patient and a haemophilia B one that cost £2.6 million have already been backed by the health assessment body.
Sources there said the earliest the Huntington's therapy could possibly be expected to be approved is the first half of 2027.
But that's only if everything else goes smoothly - and there are more hurdles to overcome.
So far, just the headline findings have been released. Scientists say the full study needs to be published and assessed by independent experts to properly assess what has been achieved.
The study is also relatively small - there were just 29 participants who were followed for 36 months. This is not unusual for gene therapies, and others have made it onto the NHS off the back of small-scale trials. The case for this treatment is particularly strong given there are no current treatment options for the disease.
But even if this therapy is licensed, it is only going to help a small portion of Huntington's disease patients as it's aimed at those with early-stage symptoms or those who haven't developed any yet. The complex nature of the surgery also means only specialist centres will be able to perform it.
Some scientists have pointed out that it is not yet certain the benefits will last long-term.
But Prof David Rubinsztein, deputy director of the Cambridge Institute for Medical Research, says the scale of this breakthrough should still not be underestimated.
He says it offers "real hope" for this devastating disease and if the approach is successfully validated in the coming months it could even have broader implications for treating other neurodegenerative diseases from Parkinson's disease and MND to dementia.
Gemma appreciates there is still some way to go before Matt can benefit, but she says for the first time in a long time she can be optimistic about the future.
"It's like a dream - I can start thinking about growing old with my husband. That would mean the world to me and my children."
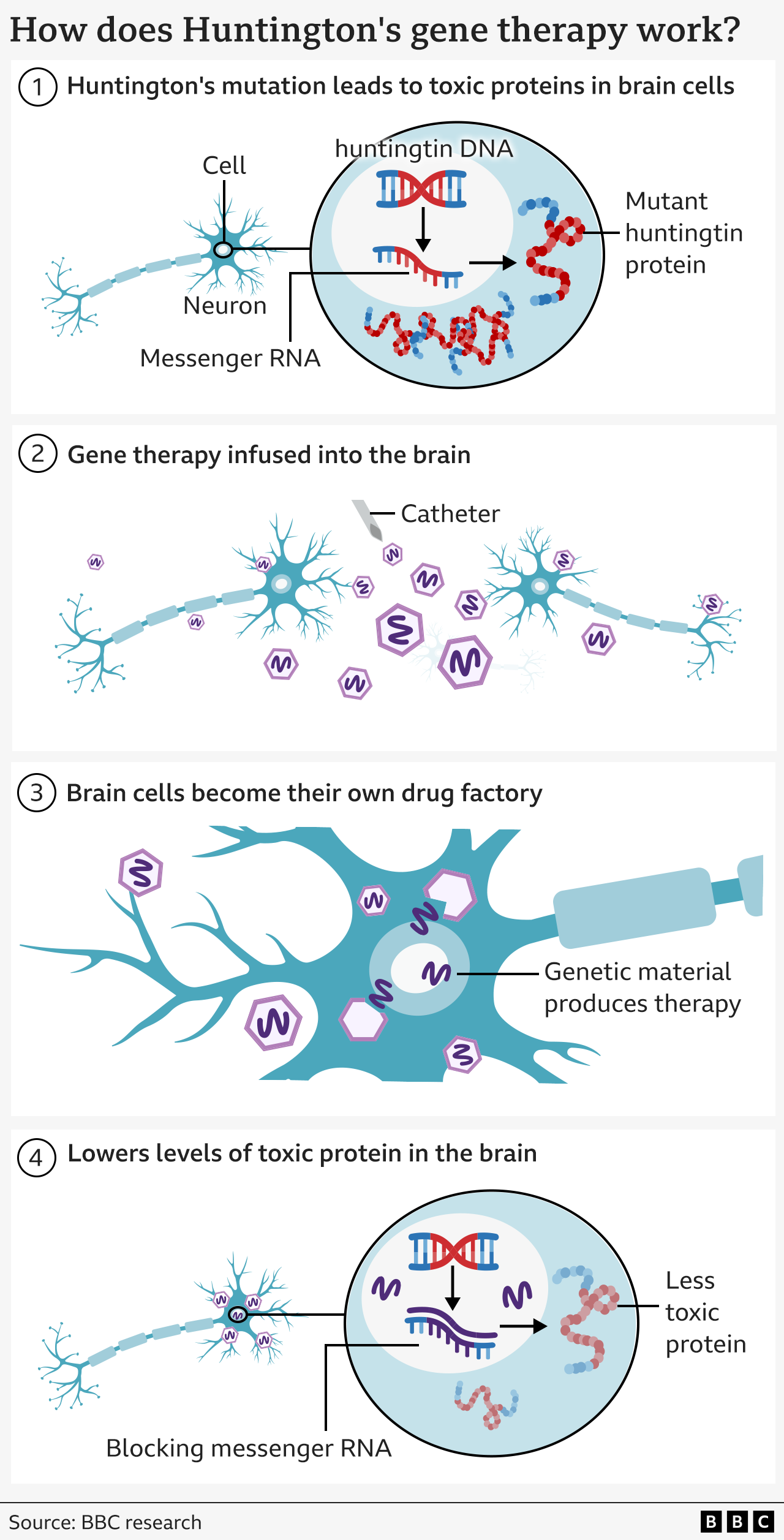
What is Huntington's disease?
Huntington's disease is caused by an error in part of our DNA called the huntingtin gene.
If one of your parents has Huntington's disease, there's a 50% chance that you will inherit the altered gene and will eventually develop Huntington's too.
This mutation turns a normal protein needed in the brain – called the huntingtin protein – into a killer of neurons.
The goal of the treatment is to reduce levels of this toxic protein permanently, in a single dose.
The therapy uses cutting edge genetic medicine combining gene therapy and gene silencing technologies.
Related topics
- Published23 January 2018

- Published16 April

- Published21 February

