WHO flags regulation gaps after India child deaths from cough syrups
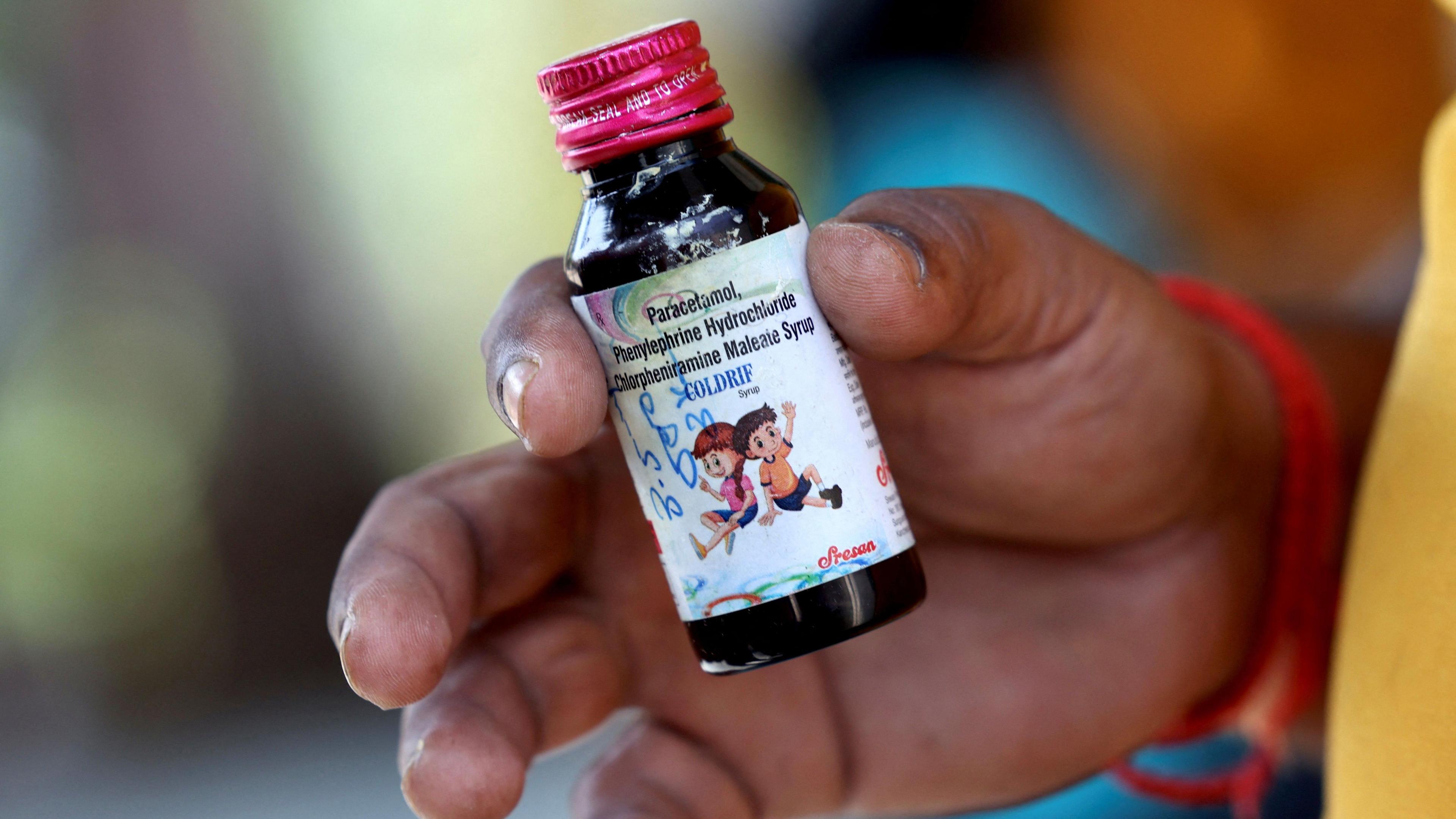
A majority of the deaths have been liked to Coldrif, an oral cough medication
- Published
The World Health Organization (WHO) has voiced "deep concern" over gaps in India's drug safety regulations, following the deaths of at least 20 children from contaminated cough syrups.
It has also warned that such medicines could reach other countries through unregulated distribution channels.
The deaths, reported from Madhya Pradesh and Rajasthan states over the past month, have been linked to three cough syrups, samples of which have been found to contain diethylene glycol (DEG) - a toxic substance found in industrial solvents.
India has arrested the owner of the pharma company behind the contaminated syrup, ordered a halt to production, external, and launched an investigation.

A health official in Tamil Nadu sticks a notice outside the Sresan Pharmaceutical factory
India's drug regulator has identified three contaminated cough syrups - Coldrif (Sresan Pharmaceuticals), Respifresh (Rednex Pharmaceuticals), and ReLife (Shape Pharma) - and shared the information with WHO.
Many states have banned these cough syrups while some have prohibited the use of all cough and cold syrups for children under the age of two.
On Thursday, police arrested G Ranganathan, owner of Sresan Pharmaceuticals. Mr Ranganathan, 73, is well-known in pharmaceutical circles and has been manufacturing medicines for decades.
Tamil Nadu Health Minister Ma Subramaniam said, external that the firm's manufacturning licence was going to be "permanently cancelled".
The deaths have made national headlines and become a subject of concern for many parents as it's a common practice in India to administer oral syrups to children.
Most deaths have occurred in Madhya Pradesh among children under five, linked to Coldrif syrup, which reportedly caused fever, vomiting, urinary problems, and rapid death.
Praveen Soni, the doctor who prescribed the syrup, has been arrested for negligence, though Indian medical groups blame regulators for inadequate testing and oversight.

A mother mourns the death of her child linked to Coldrif cough syrup
A Tamil Nadu Drug Control department inspection has found that Sresan Pharmaceuticals violated 364 manufacturing rules - 39 "very serious" and 325 "major."
The report also cited poorly qualified staff, substandard water and equipment, lack of pest control, missing production monitoring procedures, and no quality assurance or data collection department.
"Manufactured products are stored in a very unhygienic manner...Sewage was discharged without purification. Water for drug production was stored in an unhygienic manner," the report states.
Indian-made cough syrups have come under global scrutiny in recent years.
In 2023, Indian syrups tainted with diethylene glycol were linked to the deaths of 70 children in The Gambia and 18 in Uzbekistan.
Between December 2019 and January 2020, at least 12 children under five died in Jammu in Indian-administered Kashmir allegedly from cough syrup, with activists suggesting the number of casualties might have been higher.