Scientists to launch human tests of Marburg virus vaccine
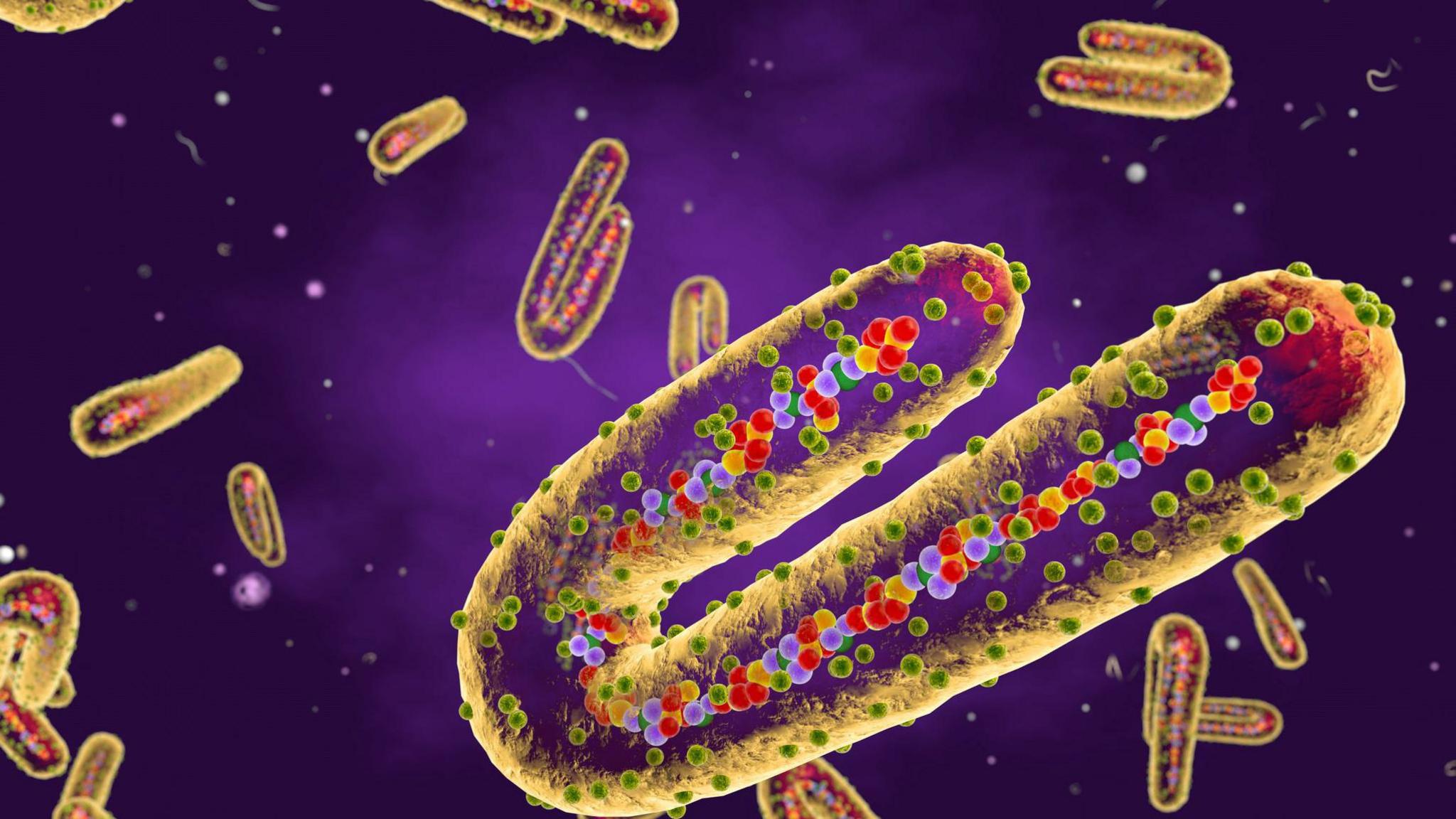
An illustration of the Marburg virus which was first discovered in 1967
- Published
Scientists are launching their first in-human vaccine trial for a highly fatal virus.
The Marburg virus is in the same family as Ebola and was discovered in 1967.
There are currently no approved vaccines or treatments for the virus, which is listed as a "high consequence" infectious disease by the UK Health Security Agency.
Now, researchers are looking for 46 volunteers to receive doses of the ChAdOx1 Marburg vaccine at the University of Oxford.
The Oxford Vaccine Group is looking to trial the vaccine on people aged between 18 and 55.
It is being funded by the Department of Health and Social Care as part of the UK Vaccine Network (UKVN), a UK Aid programme developing vaccines for diseases with epidemic potential in low and middle-income countries.
Professor Teresa Lambe OBE is lead scientific investigator, she said: “Although outbreaks of Marburg virus have historically been small, this devastating disease has started to spread even further and the potential to cause a pandemic and inflict suffering on many is a real concern."
There have been multiple outbreaks of the disease across Sub-Saharan Africa, including Tanzania and Equatorial Guinea in 2023.
The virus can be transmitted between humans through direct contact and bodily fluids which means health workers are most likely to get infected.
It can hamper the blood's ability to clot which could result in internal bleeding and can also lead to inflammation of the brain.
It has an estimated case fatality rate of up to 88% based on previous outbreaks.
Professor Lambe said: “With no approved treatments for Marburg, developing a vaccine is critical.
"This Oxford trial is a first step towards developing a safe and effective vaccine to protect people from future outbreaks.”
Follow BBC South on Facebook, external, X (Twitter), external, or Instagram, external. Send your story ideas to south.newsonline@bbc.co.uk, external or via WhatsApp on 0808 100 2240, external.
- Published22 March 2023
- Published30 September 2024
- Published18 July 2022
- Published10 August 2021
