Volunteers aim to give a knockout jab to norovirus
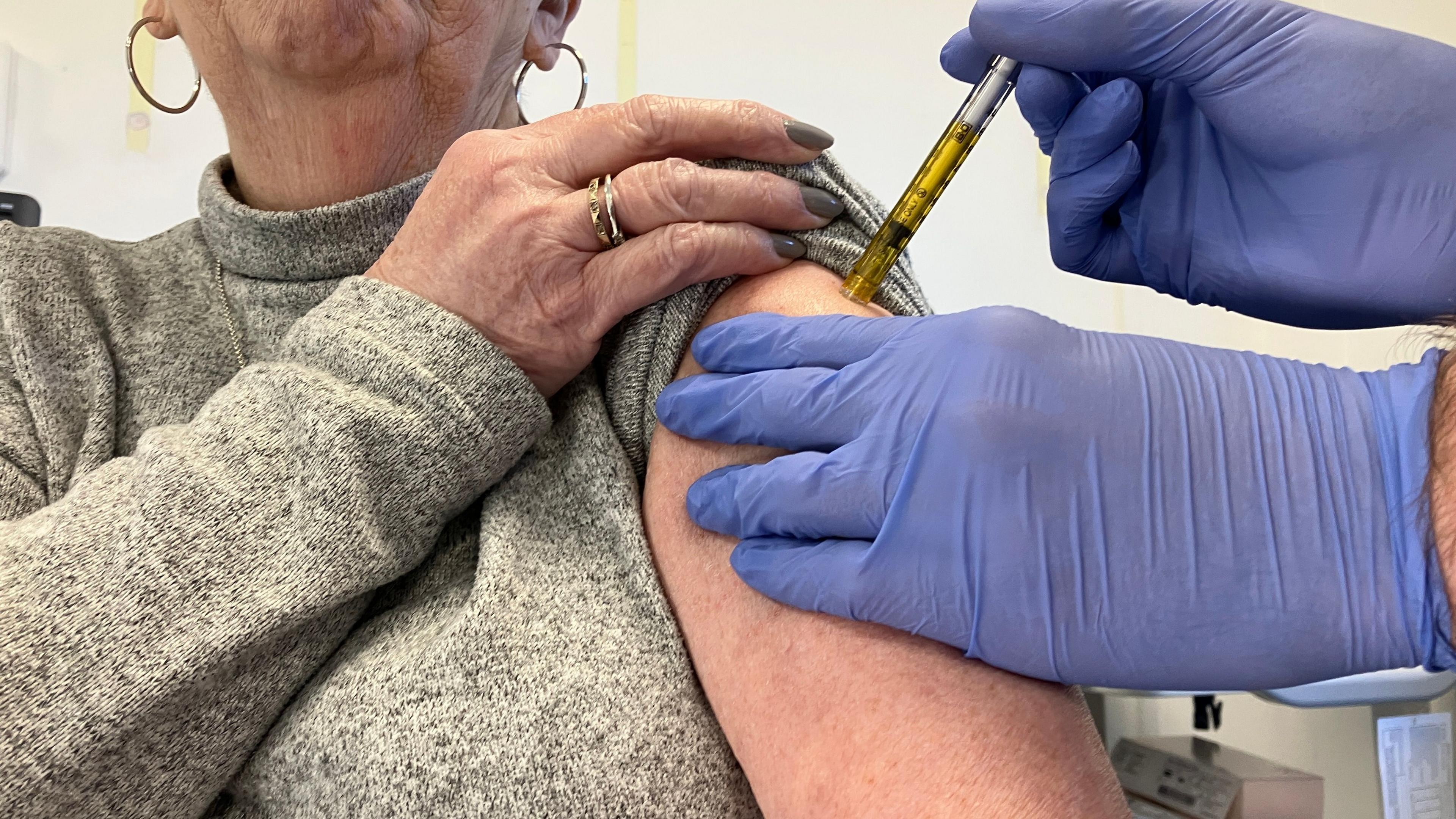
The North Wales Clinical Research Centre is taking part in a global norovirus vaccine trial
- Published
Volunteers in north Wales are among 25,000 people taking part in a global trial to find a vaccine against the symptoms of the winter vomiting bug, norovirus.
The virus affects about four million people in the UK every year, costing the NHS about £100m.
Recorded cases of the virus were up 12% in Wales this winter compared with last year, according to figures from Public Health Wales.
Doctors hope a vaccine would mean fewer hospital admissions and fewer wards being closed to prevent spreading.

Trial volunteer June Price says she wants to help people
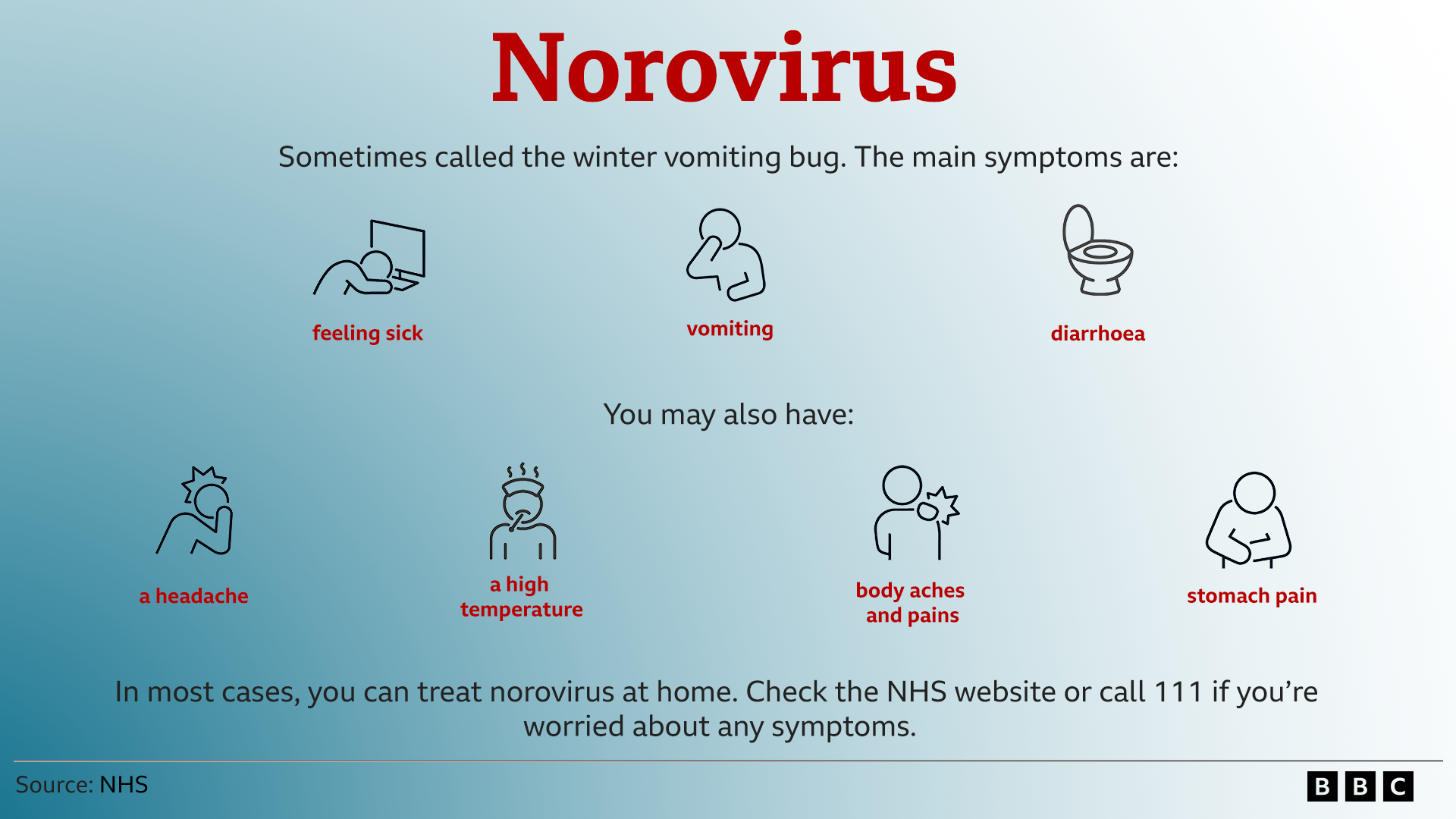
Norovirus is one of the most common causes of stomach bugs and can cause diarrhoea and vomiting.
It is particularly active during the winter months causing outbreaks in hospitals, nursing homes and nurseries.
Elderly and vulnerable people can develop severe symptoms.
June Price, 75, from Cheshire, is one of the volunteers taking part in the Nova 301 trial at the North Wales Clinical Research Centre (NWCRC) in Wrexham.
She underwent a variety of health checks to make sure she was eligible and was given an injection which could be either the vaccine or a placebo.
She said: "I think we should we should be involved because how else would they find things like the flu vaccine?
"I think people have helped me in the past, people that I don't know.
"So I would like to help people in the future."
The team at NWCRC will monitor the volunteers over the next two years and record any changes in their health.
The vaccine is being developed by pharmaceutical firm Moderna, which has recruited 25,000 volunteers worldwide, including 2,500 in the UK, for the trial.
Like Moderna's vaccine used in the Covid-19 vaccination campaign, the study involves messenger ribonucleic acid (mRNA).
When used in vaccines, mRNA shows the body's cells a bit of genetic code from the bacterium or virus.
This does not cause infection but can teach the body how to defend itself.
The body reads and translates the code and its own natural immune cells take over.

Norovirus costs the NHS £100m per year, says Dr Orod Osanlou
Dr Orod Osanlou, director of NWCRC, who is leading the trial in north Wales, said: "mRNA are fragments that the immune system can pick up so that if you are exposed to norovirus hopefully your body will be able to react more quickly and more effectively to help prevent people becoming unwell."
Dr Osanlou said norovirus costs the NHS in the UK "in excess of £100m" annually.
If the trial is successful, the Medicines and Healthcare products Regulatory Agency (MHRA) will decide whether it can be licensed.

Lynne Grundy says participants in the trial had a positive experience
Lynne Grundy, associate director for research and development at Betsi Cadwaladr Health Board, said people were generally "really pleased" to take part in clinical trials.
"We do a lot of research. We are committed to it because if we do not do research and we do not develop these treatments and these vaccines we will not be able to improve the care for patients."
Related topics
- Published23 January

- Published23 October 2024

- Published21 December 2024
