Europe's first embryonic stem cell trial at Moorfields
- Published
- comments
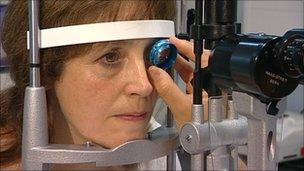
Julia Hawkins has Stargardt's disease
From the age of five Julia Hawkins has been losing her sight. She has Stargardt's disease, external, a form of macular degeneration.
Her central vision has almost entirely disappeared, leaving her with only peripheral vision.
She has to use a magnifier to read - but the vision loss does not prevent her getting about.
She negotiated the train and London tube network on Thursday to get to Moorfields Eye Hospital in London from her home in Berkshire.
British regulators have given Moorfields approval to begin trials using retinal cells derived from human embryonic stem cells (hESCs).
Excitement
Twelve patients with Stargardt's disease will have the cells injected into the eye. You can read more about the trial here.
Although there is great excitement about the trial, Julia knows that the initial phase will simply check safety.
Larger doses of the retinal pigment epithelium (RPE) cells would be used in later studies only if the treatment was deemed to be safe.
Caution
"It would be marvellous if I could get some of my sight-loss reversed", said Julia. "Even if it simply halts the deterioration, that would be great. And the real benefit would be for children. It could mean they don't need to lose any sight and have normal vision."
But doctors are urging caution. They are aware that there has been a lot of hype surrounding human embryonic stem cells, because of their potential to turn into any of the 200 or so cell types in the body.
Talk of replacing diseased tissue and even growing whole replacement organs has been commonplace.
But it is only now that the therapies are being tested in patients.
In California, the bio-tech firm Geron has begun safety trials, external of hESCs-derived cells in patients with spinal injuries.
The Moorfields trial is a partnership with another American firm, Advanced Cell Technology, external, which has already treated some patients with Stargardt's disease.
Controversial
It is always worth pointing out that other forms of stem cell treatments have been used successfully for many years.
Bone marrow transplantation is a well-established and proven technology.
More recently, stem cells from a patient's own bone marrow have been used to help create them a new windpipe.
Contrast that with human embryonic stem cells, which have yet to prove themselves in patient trials. Which is why the Moorfields study is potentially important in testing this technology.
It remains controversial. The creation of a stem cell line this way begins with the destruction of a donated human embryo at the blastocyst stage, when it is a small clump of cells.
The cell line that results is, in effect, "immortal", in the sense that it can continue to produce cells indefinitely.
Those opposed to embryo research on moral or religious grounds would prefer research on adult-derived stem cells, such as those found in bone marrow.
Scientists can also now create stem cells without destroying embryos, so-called induced pluripotent stem (iPS) cells but these have yet to make it into patients.
Significant
Josephine Quintavalle from Comment on Reproductive Ethics said: "That vulnerable UK patients will be used in highly experimental treatment which would be prohibited in most of the rest of the world is not something to triumph. Of course we must look for cures for degenerative eye diseases, but not if the unethical trade-off is the destruction of viable human embryos."
Scientists involved in this new field of medicine believe the Moorfields trial is a significant moment.
Chris Mason, Professor of Regenerative Medicine at University College London points out that it was only 13 years ago that scientists discovered a method to derive stem cells from human embryos and grow them in the laboratory.
He said: "New therapies can often take 10 or 20 years to get to market, so we are about half-way through the process".
Professor Roger Pederson, Professor of Regenerative Medicine, University of Cambridge, was also upbeat.
He said: "These stem cells are capable of forming all body tissues, and it is possible that the retinal cells formed from them will be useful in preventing age-related blindness as well as in treating certain genetic eye diseases. I believe we will now see increasing numbers of such trials in the US and UK."
So after all the talk about human embryonic stem cells, before too long we should have hard evidence as to whether the hype and the promise have been justified.
- Published22 September 2011