Watchdog NICE says no to eye drug Lucentis for diabetes
- Published
The drug is injected into the patient's eye
A drug that could save the sight of people with diabetes will not be made available on the NHS in England and Wales, an advisory body has concluded.
The National Institute for Health and Clinical Excellence (NICE) says ranibizumab, sold under the brand name Lucentis, is too expensive to use in people with diabetic macular oedema.
Charities say they will continue to campaign for the drug to be used.
At least 50,000 people in the UK are affected by this eye condition.
Sight saver
Macular oedema occurs when fluid leaks from the small blood vessels in the eye.
The fluid collects in the central part of the retina at the back of the eye, called the macular area, which can lead to severe visual impairment.
Straight lines may appear wavy and people can have blurred central vision or sensitivity to light.
Sight can become so impaired that the person can no longer read, work or drive.
Laser treatment has been the standard treatment for diabetic macular oedema on the NHS, but this only stops vision from deteriorating further.
An injection of Lucentis in the eye, however, can improve vision.
NICE already recommends Lucentis to the NHS for a different eye condition called wet age-related macular degeneration.
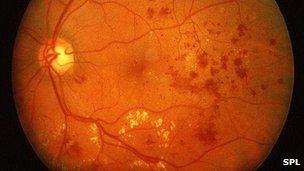
An eye examination can reveal the problem
Four UK charities - Diabetes UK, the Juvenile Diabetes Research Foundation, the Macular Disease Society and the Royal National Institute of Blind People (RNIB) - are urging government to rapidly agree a Patient Access Scheme with the manufacturer of Lucentis, Novartis, in order to bring down the cost of the drug to the NHS for treating diabetic macular oedema.
Currently, the drug costs £742.17 per injection.
Steve Winyard from the RNIB said: "We now hope that a patient access scheme can be agreed swiftly, so that patients with diabetic macular oedema are not left to needlessly lose their sight."
A spokeswoman for Novartis said the company would continue to work with NICE and the Department of Health to "ensure appropriate patients are able to receive this very important treatment, which in clinical trials has been shown to double the likelihood of gaining vision and reduce the chance of losing vision by up to three-fold compared to laser treatment".
Novartis believes that NICE did not consult sufficiently with clinical and patient experts on the data it submitted to the appraisal committee.
But Sir Andrew Dillon, Chief Executive at NICE, said the manufacturer significantly underestimated the cost of treatment.
- Published11 June 2010