Ebola trial volunteer immunised
- Published
- comments
Ruth Atkins, the first of 60 volunteers, explains why she is taking part
The first healthy volunteer has received an experimental Ebola vaccine in Oxford.
Ruth Atkins, an NHS communications manager said afterwards she felt "absolutely fine".
She is the first of 60 volunteers who will receive the jab in Oxford, with further trials due to begin in Africa next month.
Ms Atkins heard about the need for volunteers while driving home listening to BBC Radio Oxford.
She said: "I volunteered because the situation in West Africa is so tragic and I thought being part of this vaccination process was something small I could do to hopefully make a huge impact. "
The vaccine contains only a small portion of genetic material from the virus, so it cannot cause the disease.
Ms Atkins said she was not worried about safety, but had had to reassure her 15-year-old son who initially thought she was being given Ebola and would die.
Fast-track
Normally it would take years of human trials before a completely new vaccine was approved for use.
But such is the urgency of the Ebola outbreak in West Africa that this experimental vaccine is being fast tracked at an astonishing rate.
If the trials are successful, it could be used to immunise health workers in affected areas by the end of the year.
By then, around 10,000 doses should be available, and will be used to protect health workers in the worst affected regions of West Africa.
The vaccine is being developed by GlaxoSmithKline and the US National Institutes of Health.
Immune response
Funding for the trials is coming from the Wellcome Trust, Medical Research Council, and the UK Department for International Development.
The Oxford study will aim to establish two things: that the vaccine produces a good immune response in volunteers and with few side-effects.
Prof Adrian Hill: "There is absolutely no risk of this vaccine giving anyone Ebola"
The vaccine uses a modified chimpanzee common cold virus to carry a single Ebola protein - it cannot trigger either disease, but should prompt the production of antibodies against Ebola.
Professor Adrian Hill, director of the Jenner Institute in Oxford, who is leading the trial, said: "This is a remarkable example of how quickly a new vaccine can be progressed into the clinic, using international co-operation".
Blood tests from the volunteers will reveal the extent of their antibody response within two to four weeks.
Further volunteers will be given the vaccine in Africa next month and there are trials in the US of a different formulation - both vaccines could be used if they prove safe and effective.
Promising results from animal studies were published earlier this month., external
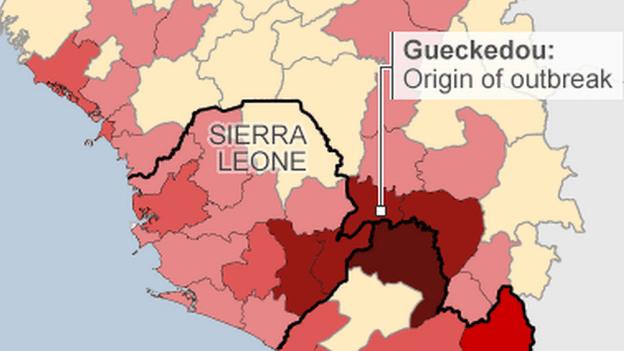
The US has said it will send 3,000 troops to West Africa to build treatment clinics and to train health workers in how to halt the spread of the deadly virus.
The official death toll now stands at more than 2,400 people, although the outbreak may have killed many times that number.
- Published17 September 2014
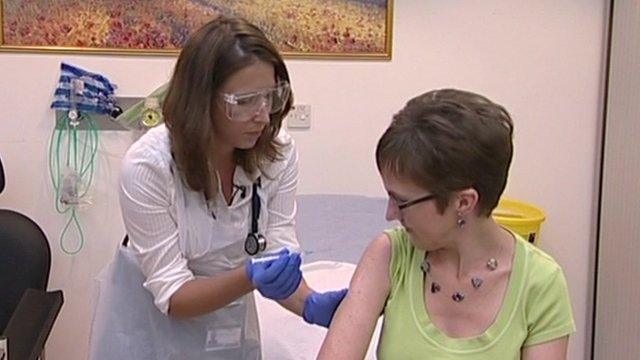
- Published17 September 2014

- Published21 August 2014

- Published14 January 2016