Cancer immunotherapy approved in UK
- Published
Melanoma, the most serious form of skin cancer, kills more than 2,000 people a year in Britain
A pioneering cancer drug that harnesses the power of the immune system has been approved for use in the UK.
Nivolumab was one of the drugs labelled a "milestone" therapy at a major cancer conference last month.
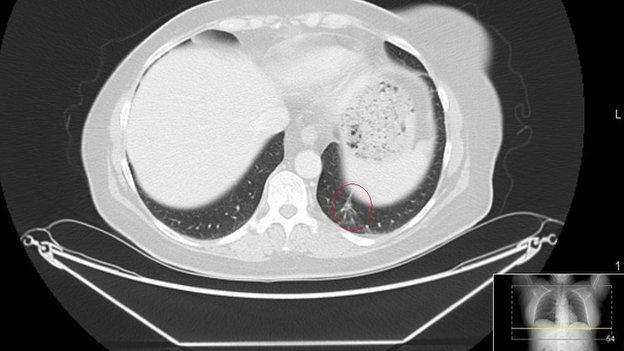
It has been approved for lung cancer through the UK's Early Access to Medicines Scheme and has been given an EU license for melanoma.
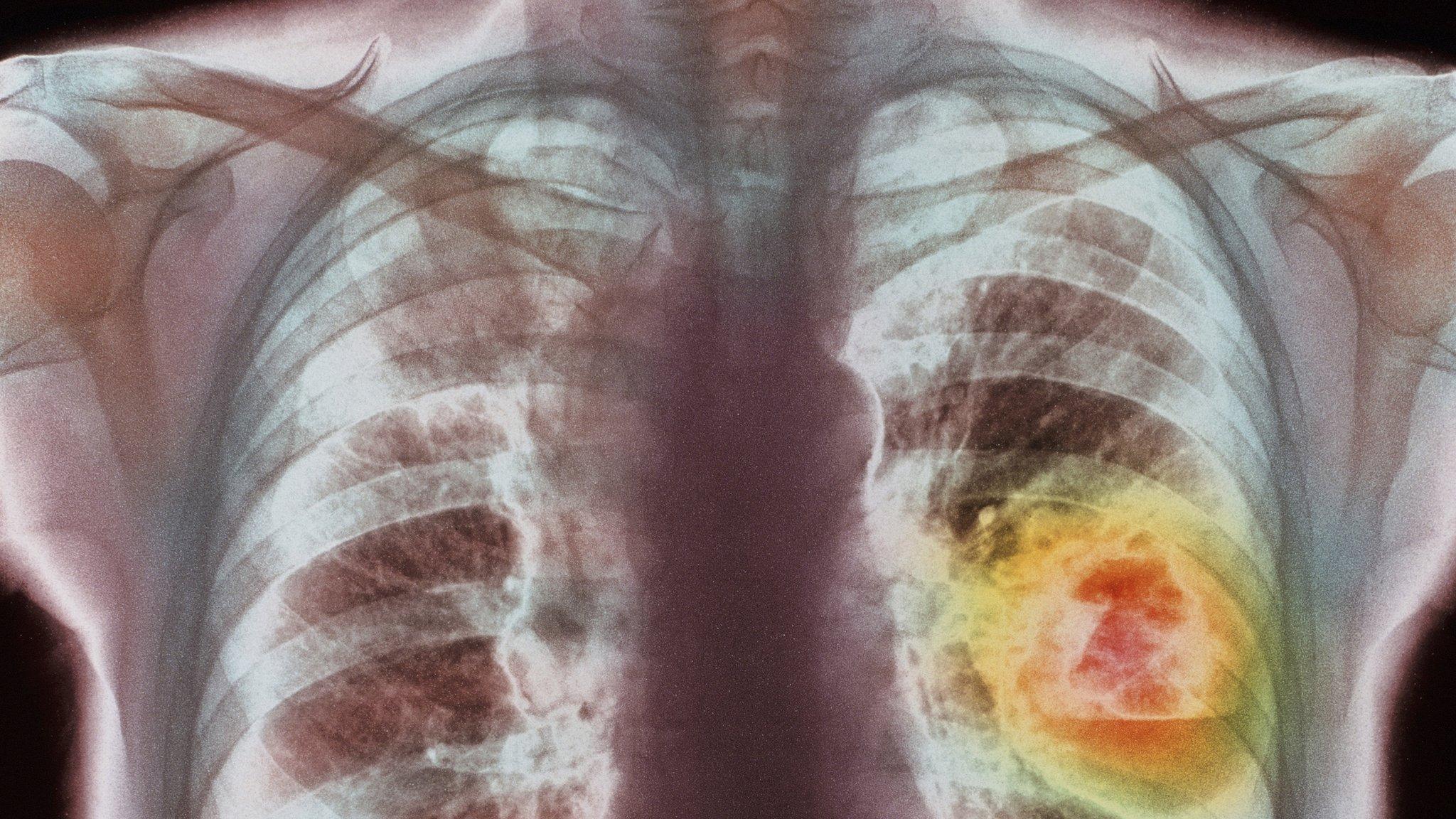
The drug has been shown to stop skin and lung cancers from progressing, in clinical trials.
Enhanced defence
The immune system is a powerful defence against infection. However, there are many "brakes" built in to stop the system attacking its own tissues.
Cancer - which is a corrupted version of healthy tissue - can take advantage of these brakes to evade assault from the immune system.
Nivolumab takes the brakes off.
One trial of nivolumab, alongside an already approved medicine ipilimumab, stopped melanoma advancing for nearly a year in 58% of patients.
A separate trial in lung cancer showed the drug more than doubled survival times in some patients.
The treatment has now been allowed in the UK for both skin and lung cancers.
Gill Nuttall, from Melanoma UK, said: "There has been an alarming rise in the number of cases of melanoma in the UK over recent years.
"Today's news is therefore very welcome as it provides more options for patients and the potential of better, longer survival."
- Published30 May 2015
- Published1 June 2015