Essure implant study finds safety concerns
- Published
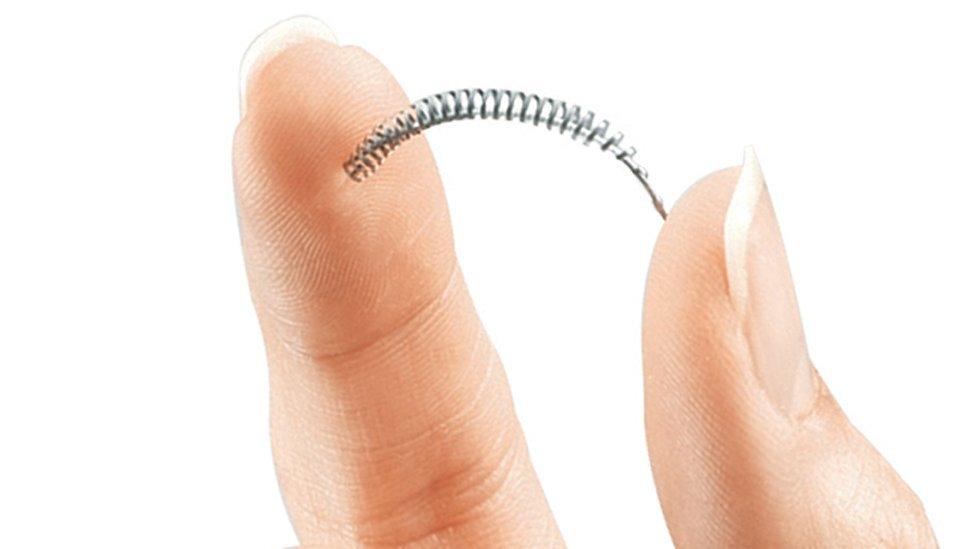
An implant used by the NHS to sterilise women poses a serious safety concern, according to a new US study in the BMJ.
Women who had Essure fitted had a 10-times greater risk of needing a repeat operation than women who had conventional sterilisations.
Regulators say the device is safe but are monitoring the situation following complaints from users about side-effects such as pain and misplacement.
Manufacturer Bayer says Essure's safety and efficacy are backed up by evidence.
Essure is a spring that doctors insert into a woman's fallopian tubes to block them and stop eggs reaching the womb.
The UK's Medicines and Healthcare products Regulatory Agency (MHRA) says it will continue to monitor all side-effects and advises UK women who experience problems to see a doctor. But it says the device - given to about 1,500 women a year in the UK - is safe to use.
The research in the British Medical Journal, external tracked 8,000 women who had Essure fitted and 44,000 women who underwent conventional "tube tie" sterilisation to compare how well the procedures worked.

Female sterilisation
There are different procedures but they work in a similar way - by preventing eggs from travelling down the fallopian tubes so that fertilisation can't happen.

The fallopian tubes can be cut, clipped or blocked from the inside
Conventional sterilisation involves a small incision in the abdomen so that the fallopian tubes can be cut and sewn shut or closed off using clips or rings.
Essure doesn't require an incision. Instead, the device is guided into place through a narrow tube instrument via the vagina.
Both methods should be considered irreversible and permanent.

The unintended pregnancy rate was low for both. But more women who had Essure experienced complications that meant they needed repeat surgery.
Although rare, there have been instances of the device puncturing neighbouring organs, such as the bowel, or migrating around the body.
Other women have reported nasty side-effects, such as pain or allergy to nickel found inside the implant.
In the study, Essure was linked to an extra 21 repeat operations per 1,000 patients undergoing surgery, which the authors say is significant and "a serious safety concern".
Study author Dr Art Sedrakyan said: "It may not sound many but this is a commonly done procedure so that makes it relevant.
"Our study is very helpful. It shows how Essure compares with the main alternative, which means women can weigh the benefits and harms and regulators can make better decisions."
Bayer points out that because all women who have Essure receive a routine check-up three months after having the device fitted, this may partly explain why more in the study had follow-up surgery.
"Because there is no confirmation test that could identify potential failure of a laparoscopic tubal ligation procedure, it stands to reason that the comparative reoperation rate would be lower," said a spokeswoman.
Adverse incidents can be reported to the MHRA on its website, external or by calling 020 3080 7080. In the US, patients should contact the Food and Drug Administration, external.
- Published4 October 2015
- Published24 June 2014
