Dawn of gene-editing medicine?
- Published
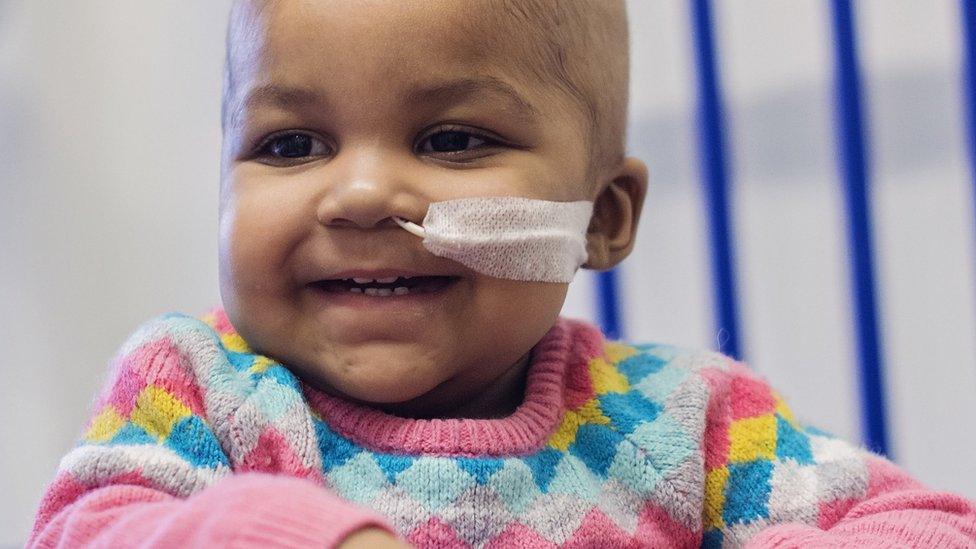
Does the smiling face of Layla Richards mark a new era in genetic medicine that could change all our lives?
Her story is simply remarkable and a world first.
On the day before her first birthday, Layla's parents were told that all treatments for her leukaemia had failed and she was going to die.
The determination of her family, doctors and a biotechnology company led to her being given an experimental therapy that had previously been tried only in mice.
Now, just months after her family was told her cancer was incurable, Layla is not only alive, but a happy, giggling child with no trace of leukaemia in her body.
The "miracle" treatment was a tiny vial filled with genetically engineered immune cells that were designed to kill her cancer.
Layla's mother, Lisa: "There's always got to be a first... we begged them to try something"
There's no doubt this is exciting stuff and it raises questions about the future of medicine.
There is already talk of a revolution - of using similar techniques to treat a range of cancers, but also inherited diseases such as sickle cell anaemia.
But we have been here before.
Around the turn of the millennium, over-excited scientists and journalists were proclaiming that gene therapy was going to transform the world.
It hasn't happened, so has the "miracle" really changed anything?
Prof Adrian Thrasher, from Great Ormond Street Hospital, told me: "There was a lot of hype that was unrealistic at the time, the technologies were very new and it's taken 15-20 years for those technologies to mature.
"I think we're seeing the fruits of those early studies right now, so I think this is real."
DNA
All types of gene-based therapy involve changing the blueprint of life - our DNA, which contains the instructions for building and running every part of the human body.
In the early incarnations of these therapies, new DNA was inserted into the cells of patients who had missing or defective instructions in their DNA.
The most famous cases were boys with so-called bubble boy syndrome in the 1990s.
They had no immune system and had to live in completely sterile conditions due to a defect in a gene called IL2RG.
This was successfully replaced by using a virus to "infect" cells with a healthy copy of the DNA, but ultimately trials were abandoned after patients developed leukaemia, external.
The problem was the DNA was being inserted almost at random and in such a way that it disrupted the natural functioning of some cells and they became cancerous.

DNA is our blueprint of life
What has happened since then is precision.
The viruses being used can place DNA into safer sites in the genome and three key technologies have arrived on the scene.
Zinc fingers, Talens and Crispr all share the same general concept - they act as a type of satnav that finds its way to specific sites in our DNA and a pair of molecular scissors that can edit the DNA.
They have opened up a whole new field - genetic engineering - in which not only can new information be inserted, the code of life that is already there can be rewritten.
This is exactly what happened in Layla's case.
White blood cells were taken from a donor. Talens were used to engineer protection against anti-cancer drugs being given to the patient and to stop them attacking healthy tissue.
And a virus was used to insert a new gene that would make it attack leukaemia cells.

Will doctors be able to harness our DNA for new treatments
Zinc fingers are already being tested in HIV-positive patients. The aim is to take the patient's cells out of the body, give them HIV protection, and then put them back in.
Crispr has attracted its own controversy after being used to edit the DNA of an embryo for the first time.
Prof Waseem Qasim, who was involved in treating Layla's leukaemia, told the BBC: "The technology is moving very fast, the ability to target very specific regions of the genome has suddenly become much more efficient.
"The technology itself has got enormous potential to correct other conditions where cells are engineered and given back to patients or to provide new properties to cells that allow them to be used in a way we can only imagine at the moment."
Re-arming the immune system to target cancer and a wide range of inherited disorders is in the sights of doctors.
It will be easiest for them to use cells that can be taken out of the body, modified and reinserted rather than trying to edit them still in the body.
So diseases of the blood or immune system - such as beta thalassaemia or sickle cell anaemia - will be easier targets than kidney or heart defects.
Prof Thrasher is predicting an "explosion" in the use of such genetic engineering in the next 10 years.
He argues: "In the past, we had treatments for a very small number of patients and now it's going into mainstream medicine.
"We're at the point now where we can treat many more patients, we can see the breadth of this expanding and to me, that's the exciting part."
After the overhyped false dawn fifteen years ago, gene-editing is now, it seems, about to arrive.
Follow James on Twitter, external.
- Published5 November 2015
- Published13 May 2015
- Published19 January 2015
.jpg)