New treatment can 'halt' multiple sclerosis, says study
- Published
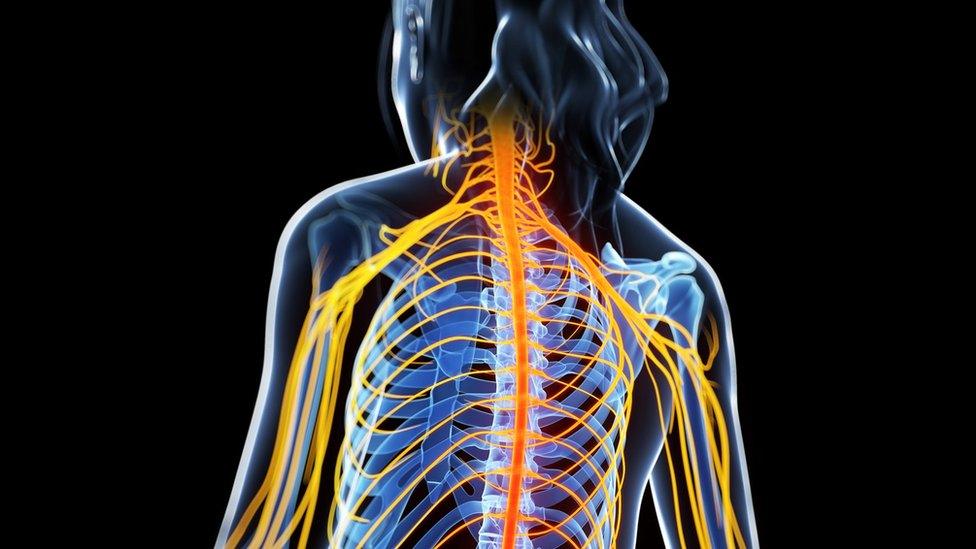
MS causes the body's own immune system to attack the lining of nerves in the brain and spinal cord
Aggressive chemotherapy followed by a stem cell transplant can halt the progression of multiple sclerosis (MS), a small study has suggested.
The research, published in The Lancet,, external looked at 24 patients aged between 18 and 50 from three hospitals in Canada.
For 23 patients the treatment greatly reduced the onset of the disease, but in one case a person died.
An MS Society spokeswoman said this type of treatment does "offer hope" but also comes with "significant risks".
Around 100,000 people in the UK have MS, which is an incurable neurological disease.
'No relapses'
The condition causes the immune system to attack the lining of nerves in the brain and spinal cord. Most patients are diagnosed in their 20s and 30s.
One existing treatment is for the immune system to be suppressed with chemotherapy and then stem cells are introduced to the patient's bloodstream - this procedure is known as an autologous haematopoietic stem cell transplant (HSCT).
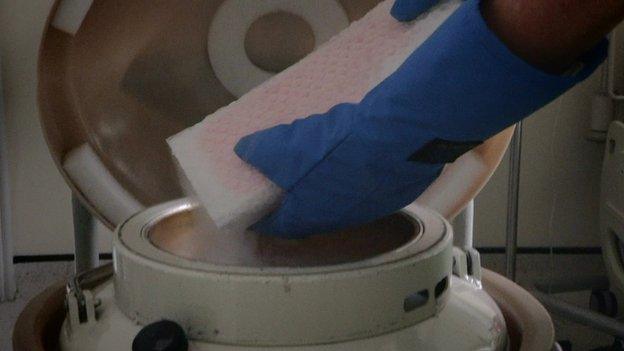
An MS patient's stem cells being removed from the freezer prior to transplant
But in this study, Canadian researchers went further - not just suppressing the immune system, but destroying it altogether.
It is then rebuilt with stem cells harvested from the patient's own blood which are at such an early stage, they have not developed the flaws that trigger MS.
The authors said that among the survivors, over a period of up to 13 years, there were no relapses and no new detectable disease activity.
All the patients who took part in the trial had a "poor prognosis" and had previously undergone standard immunosuppressive therapy which had not controlled the MS - which affects around two million people worldwide.
One person died as a result of the strong effects of the chemotherapy, the authors said.
'Disease free'
Lead author Dr Mark Freedman admitted there were limitations to the study - such as the small sample size - and there was no control group used for comparison with those who were treated.
He said: "Larger clinical trials will be important to confirm these results.
"Since this is an aggressive treatment, the potential benefits should be weighed against the risks of serious complications associated with HSCT and this treatment should only be offered in specialist centres experienced both in multiple sclerosis treatment and stem cell therapy, or as part of a clinical trial."
Dr Emma Gray, who is head of clinical trials at the MS Society, said: "This type of stem cell transplantation is a rapidly evolving area of MS research that holds a lot of promise for people with certain types of MS.
"This treatment does offer hope, but it's also an aggressive procedure that comes with substantial risks and requires specialist aftercare. If anyone is considering HSCT we'd recommend they speak to their neurologist."
Prof Siddharthan Chandran at the University of Edinburgh described the work as "important and carefully conducted".
"...[It] demonstrates that powerful chemotherapy-based treatment for a selected subset of MS patients with very aggressive disease is effective in preventing further disabling relapses and, in a proportion, appears to render them effectively disease-free," he said.
Meanwhile, Dr Stephen Minger, a stem cell biologist and independent consultant, described the study as "truly impressive".
He said: "It's important to stress that this is a very early study, though with impressive long-term follow-up of treated patients.
"Nevertheless, the clinical results are truly impressive, in some cases close to being curative, though we need longer-term follow-up to know for certain whether the patients continue to do well or if there is a chance of relapse."

Multiple sclerosis
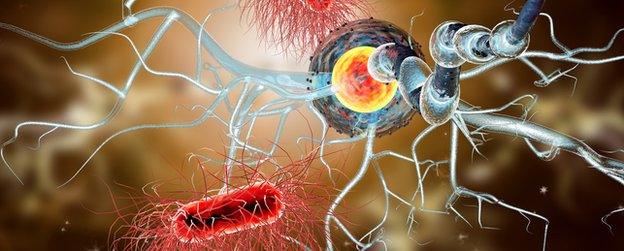
In MS the protective layer surrounding nerve fibres in the brain and spinal cord - known as myelin - becomes damaged. The immune system mistakenly attacks the myelin, causing scarring or sclerosis.
The damaged myelin disrupts the nerve signals - rather like the short circuit caused by a frayed electrical cable.
If the process of inflammation and scarring is not treated then eventually the condition can cause permanent neurodegeneration.

- Published18 January 2016
- Published18 January 2016
- Published1 June 2016
- Published28 October 2015
