Vaginal mesh operations should be banned, says NICE
- Published
The mesh is made of polypropylene - the same material used to make certain drinks bottles
The health watchdog NICE is to recommend that vaginal mesh operations should be banned from treating organ prolapse in England, the BBC's Victoria Derbyshire show has learned.
Draft guidelines from NICE say the implants should only be used for research - and not routine operations.
Some implants can cut into the vagina and women have been left in permanent pain, unable to walk, work or have sex.
One expert said it is highly likely the NHS will take up the recommendation.
However, the organisation is not compelled to act on findings it receives from NICE.
Both NHS England and NICE declined to comment.
Stephanie and Peter Williams say it's made it "impossible" for them to be intimate
'Life-changing consequences'
In the documents - to be published after consultation in December - NICE said there were "serious but well-recognised safety concerns" and that "evidence of long-term efficacy [for implants treating organ prolapse] is inadequate in quality and quantity".
It added that "when complications occur, these can be serious and have life-changing consequences", but said "most commentaries received from patients reported satisfaction with the procedure".
One woman, Margie Maguire, 41 - told the Victoria Derbyshire programme she cannot have any more children or walk unaided because of the damage caused by the mesh.
"I have chronic pelvic pain on a daily basis and I'm on nine different medications when I have a pain attack.
"These can last from two to six hours at a time and is like having a heart attack," she said.
Kate Langley told the programme in April she had been admitted to hospital 53 times to try to end the pain, but - like many women - the mesh was so near the nerve it could not be fully removed.
She has been left with nerve damage and in permanent pain by the implants, giving up her business as a childminder because the pain was so intense.
The surgeon who first examined her, she explained, "could see the [mesh] tape had come through my vagina - protruding through".

Kate Langley has been left in permanent pain by her vaginal mesh implant
The plastic meshes are made of polypropylene - the same material used to make certain drinks bottles - and manufactured by many different companies.
They are used to support organs such as the vagina, uterus, bowel, bladder or urethra which have prolapsed after childbirth.
The University of Oxford's Prof Carl Heneghan, an expert in the subject, said the draft guidelines were an admission that health services had "got this wrong" - calling the use of mesh a "catastrophe".
He described the draft guidelines as a "backdoor ban" on implants that would effectively end their use.
But he said it had come too late.

Prof Heneghan says the use of the implants has been "absolutely farcical"
"Seven years I have been watching this emerge - it is absolutely farcical how bad it is. Either they're burying their heads in the sand or they don't know what they're doing."
He called for a registry to be created for everyone who had been treated with the implants so that their effects could be fully understood.
In April, the BBC learned more than 800 UK women are taking legal action against the NHS and the makers of vaginal mesh implants.
The NICE documents suggest "randomised controlled trial data showed no added benefit of using mesh compared with native tissue repair".
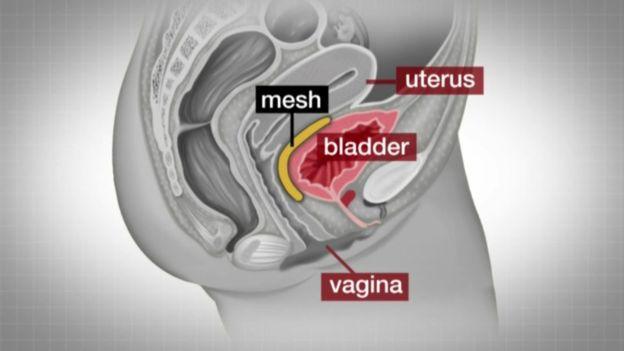
Mesh implants are used to treat organ prolapse and urinary incontinence
Between April 2007 and March 2015, more than 92,000 women had vaginal mesh implants in England, according to NHS data from the Hospital Episodes Statistics.
About one in 11 women has experienced problems, the data suggests.
The use of vaginal mesh to treat urinary incontinence is not mentioned in the draft NICE guidelines.
In Scotland, former Scottish Health Secretary Alex Neil requested a suspension of mesh implants by the NHS in 2014, but figures obtained by the BBC in December 2016 showed hundreds of operations have been performed since.
A number of Scottish health boards have stopped using mesh implants altogether.
The mesh is also used routinely in hernia repair despite concerns it is leaving many patients in chronic pain.
The Department of Health declined to comment.
Watch the Victoria Derbyshire programme on weekdays between 09:00 and 11:00 on BBC Two and the BBC News Channel.
- Published18 April 2017

- Published18 October 2017

- Published18 October 2017
