Medical cannabis products available on prescription
- Published
- comments

Charlotte Caldwell previously said the law change would mean her son, Billy, would be able to live a "normal life"
Medicinal cannabis products can now be legally prescribed to some patients across the UK for the first time.
The treatments can be prescribed only by specialist doctors in a limited number of circumstances where other medicines have failed.
The decision to relax the rules on the treatments followed an outcry over two boys with severe epilepsy being denied access to cannabis oil.
But one charity fears access to it will be "much more limited" than expected.
Is the UK the world's biggest exporter of legal cannabis?
What are the rules about cannabis oil in the UK?
Among those who stand to benefit will be children with rare, severe forms of epilepsy.
Cannabis-derived medicines: What you need to know
Who can receive the treatments?
As of Thursday cannabis-based products can be prescribed, but only by specialist hospital doctors in a small number of cases, and not by GPs.
New NHS guidance for doctors in England, external says it should be prescribed only when there is clear published evidence of its benefit and other treatment options have been exhausted.
The treatments can be prescribed in cases of
Children with rare, severe forms of epilepsy
Adults with vomiting or nausea caused by chemotherapy
Adults with muscle stiffness caused by multiple sclerosis
If a patient is not already in touch with a specialist doctor they can be referred to one by their GP if the doctor deems this appropriate.
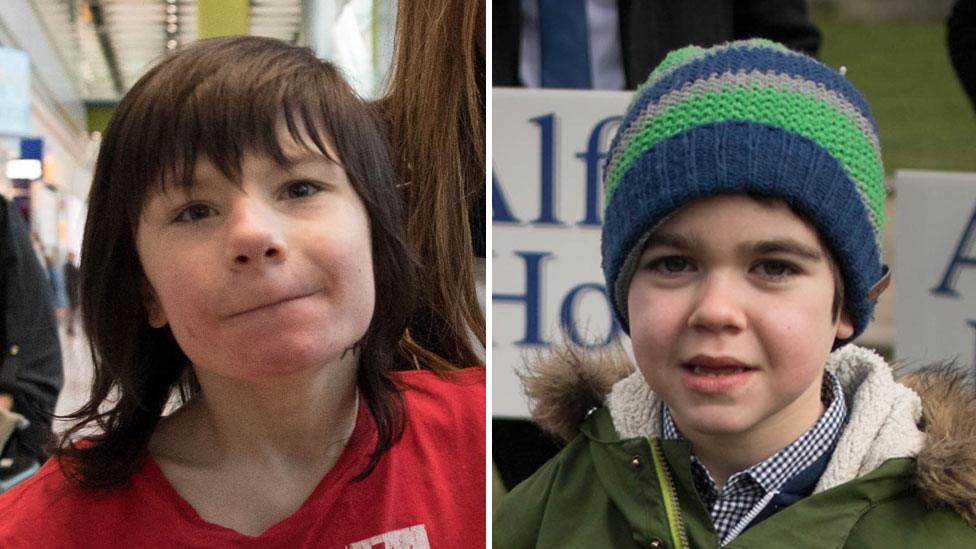
Billy Caldwell and Alfie Dingley were granted licences to allow them access to cannabis oil
Which treatments will be prescribed?
The Home Office has said the treatments must have been produced for medical use and be regulated.
In practice, experts say this is likely to include pills, capsules and oils, but not smoking cannabis.
Treatments contain varying quantities and ratios of the compounds THC, which makes people feel "high", and CBD, another compound scientists are investigating for its potential medical benefits.
It will be down to individual hospital trusts to decide how to fund the treatments.
How did we get to this point?
The decision to allow specialist doctors to prescribe medicinal cannabis products follows the high-profile cases of Alfie seven, and Billy, 13.
Both have severe epilepsy which their families say has been markedly improved by cannabis oil treatments that had not been legally available in the UK.
Initially, the Home Office refused requests for a licence for Alfie to use the oil.
Billy's mother, Charlotte, had cannabis oil that she brought back from Canada confiscated at Heathrow Airport.
Their plight prompted MPs to criticise the "bizarre and cruel" rules over medicinal cannabis.
The boys have since been granted special licences to access their treatments.
The cases prompted Home Secretary Sajid Javid to announce in June that there would be a review of medicinal cannabis.
That review, which came in two parts, concluded there was evidence medicinal cannabis had therapeutic benefits and that doctors should be able to prescribe the products, provided they met safety standards.
In July Mr Javid confirmed that specialists doctors would be able to prescribe cannabis-derived medicinal products.
What were the rules before?
Before today, almost all cannabis-based medicinal products were classed as Schedule One drugs, which means they were judged to have no therapeutic value. Sativex, a treatment containing the cannabis compounds THC and CBD, is one of the few that is already approved.
This meant these products could not be legally prescribed in the UK, and could be accessed only, in rare cases, with a special licence from the Home Office.
Now treatments that meet "appropriate standards" have been reclassified into Schedule Two - those that have a potential medical use.

The government says this is not a first step towards legalisation of recreational cannabis use, as in Canada
How have people reacted to the law change?
Alfie Dingley's mum, Hannah Deacon, said the guidelines meant people would struggle to access the oil her son had been given because scientific studies had not been carried out on the treatment.
"It's absolutely gutting and not what my campaign was about."
Emma Appleby, whose nine-year-old daughter, Teagan, has severe epilepsy, said it was not clear when she will receive medical cannabis.
She also said the guidelines suggested Teagan would be given a weaker form of treatment than doctors had initially suggested to her.
She said: "It's heart-breaking to watch your child suffer every single day, knowing there's a product that can help, could help...
"Watching my daughter suffer every day, it's horrible."
Dr Michael Bloomfield, from University College London, said the UK's approach was sensible.
"It's going to be very hard for doctors to prescribe cannabis-related products to begin with, and I think it's right that's the case.
"When we don't have very strong evidence for any medicine, then it should be hard to prescribe something because we should be prescribing medicines when there's a very strong evidence base for them."
But the MS Society said the guidance made access to treatments "much more limited than we were led to believe".
Genevieve Edwards, from the society, added: "We're calling on NHS England to revisit this guidance urgently, and engage with neurological experts to ensure people with MS are not left disappointed and unable to access the right treatment for them."
Is this a step towards recreational legalisation?
The government has been quick to say that this is not a first step towards legalising cannabis.
"There will be strict controls in place and this is in no way a step towards legalising the recreational use of cannabis," Home Secretary Sajid Javid has previously said.
An NHS spokeswoman said the change to the law "does not detract from the wider physical and mental health risks and concerns potentially arising from regular recreational cannabis use".
- Published9 October 2018

- Published19 July 2018

- Published10 July 2018

- Published5 July 2018

- Published19 June 2018
- Published26 July 2018
