Emerade adrenaline pens recalled over activation fault
- Published
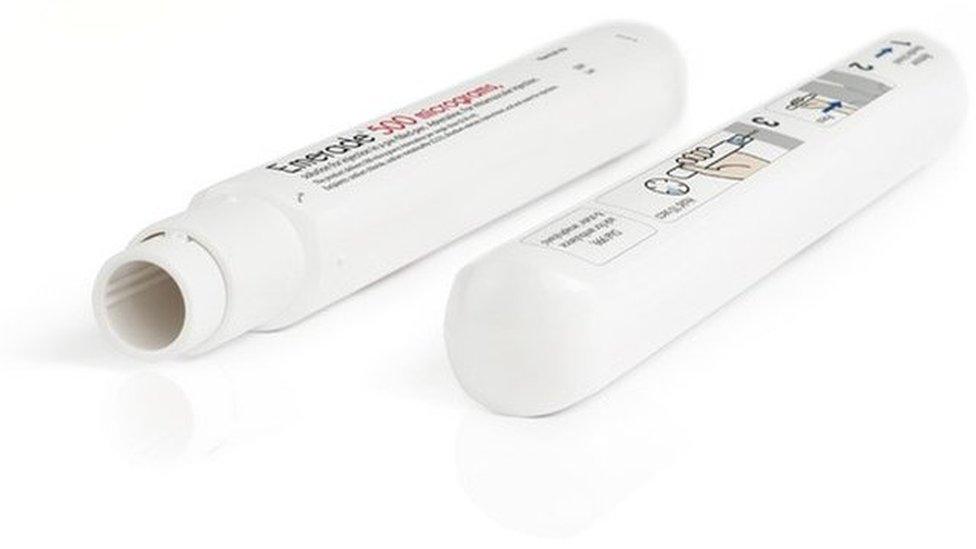
Allergy patients are being urged to return Emerade 150-microgram adrenaline pens to pharmacists because of a potential fault.
The Medicines and Healthcare products Regulatory Agency (MHRA) said some users had reported difficulty activating the needles.
The defect also affects 300- and 500-microgram pens but there are currently insufficient stocks to replace them.
Patients should keep these until the expiry date but always carry two pens.
Most of the affected pens, used for the emergency treatment of severe life-threatening allergic reactions in children, will activate normally, according to the MHRA, but manufacturers say 13% need to be applied with more force.
Trouble activating the pens was first reported last year.
There are three brands of adrenaline pens available in the UK - Emerade, EpiPen and Jext.
Reactions can be triggered by certain foods, such as nuts, fish, milk and eggs, medicines and insect stings.
Patients carrying any adrenaline pen should:
Check the expiry date and replace the pen before it expires
After using the pen, call 999 and lie flat with legs elevated to keep blood flowing
Use a second pen if still feeling unwell after five to 15 minutes
- Published12 July 2019
- Published4 March 2020