Moderna becomes third Covid vaccine approved in the UK
- Published
- comments
Prime Minister Boris Johnson has said it is "excellent news" that a third coronavirus vaccine has been approved for use in the UK.
It is made by US company Moderna and works in a similar way to the Pfizer one already being offered on the NHS.
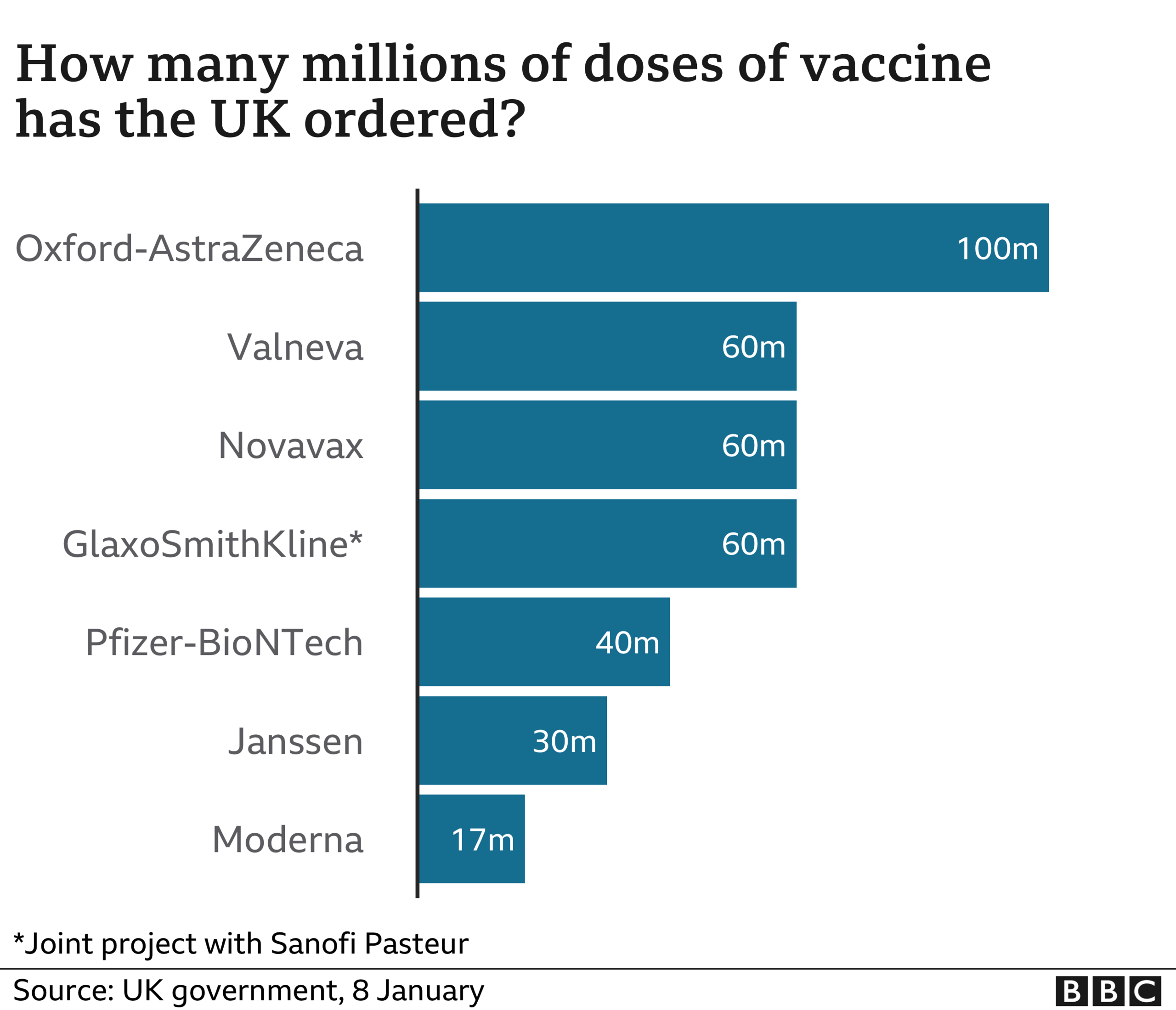
The UK has pre-ordered 17 million doses of the Moderna vaccine - 10 million more than planned - but supplies are not expected to arrive until spring.
It is the last Covid vaccine with final trial data published.
There are hundreds still in development, with some expected to report findings in the near future.
Around 1.5 million people in the UK have had at least one dose of a Covid vaccine so far, with either the Pfizer or AstraZeneca vaccines already approved by UK regulators.
That figure includes almost a quarter of those aged over 80 in England - people at highest risk of severe illness or death from the virus.
Vaccines are being given to the most vulnerable first, as set out in a list of nine high-priority groups, covering around 30 million people in the UK.
Vaccine Deployment Minister Nadhim Zahawi welcomed the approval of the Moderna jab
The prime minister has said the aim is to vaccinate 15 million people in the UK by mid-February, including care homes residents and staff, frontline NHS staff, everyone over 70 and those who are clinically extremely vulnerable.
Health and Social Care Secretary Matt Hancock said: "This is further great news and another weapon in our arsenal to tame this awful disease."
The UK had originally ordered 7 million doses of the Moderna jab, but has increased this to get even more people immunised as quickly as possible.
In total, the UK has now ordered 367 million doses of vaccines to protect against Covid-19.
Nadhim Zahawi, vaccine deployment minister, said: "The NHS is pulling out all the stops to vaccinate those most at risk as quickly as possible, with over 1,000 vaccination sites live across the UK by the end of the week to provide easy access to everyone, regardless of where they live.
"The Moderna vaccine will be a vital boost to these efforts and will help us return to normal faster."
Covid vaccine safety: How does a vaccine get approved?
The Moderna vaccine, an RNA vaccine like Pfizer's, injects part of the virus's genetic code in order to provoke an immune response.
It requires temperatures of around -20C for shipping - similar to a normal freezer.

In comparison, the Pfizer/BioNTech one requires temperatures closer to -75C, making transport logistics much more difficult.
The AstraZeneca jab is easier to store and distribute, as it can be kept at normal fridge temperature.
All of these vaccines require a second booster shot, but a first dose is likely to be given to as many people as possible.
In trials with more than 30,000, the Moderna vaccine offered nearly 95% protection from severe Covid.
No vaccine is 100% effective and it takes time for protection to build. For all of the Covid vaccines, we still do not know how long immunity will last.
People who have received a coronavirus vaccine should continue to follow social distancing rules to protect themselves and others.
EU and US regulators have already approved the Moderna vaccine.
Related topics
- Published16 November 2020
- Published28 May 2021