Covid: Large trial of new treatment begins in UK
- Published

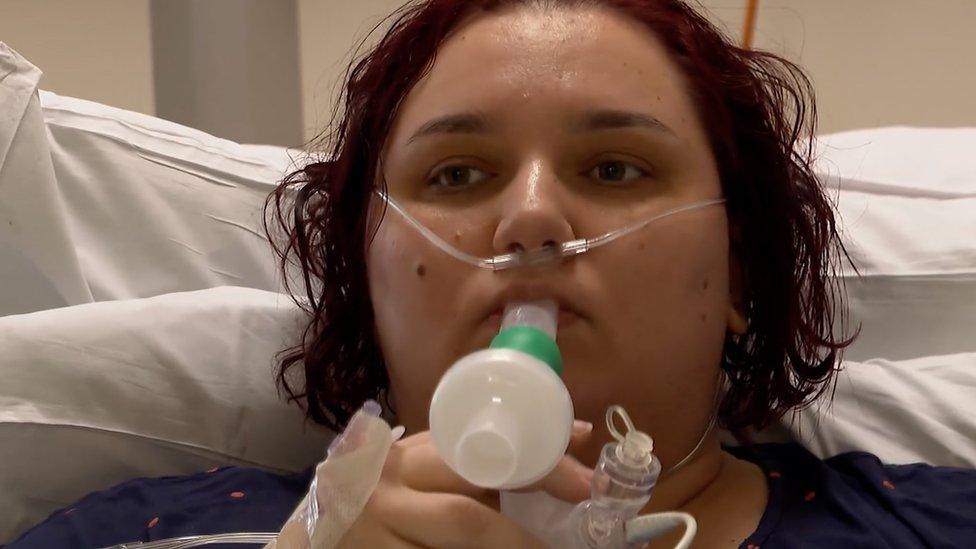
Kaye Flitney is one of those enrolled on the clinical trial
A large-scale trial of a new treatment it is hoped will help stop Covid-19 patients from developing severe illness has begun in the UK.
The first patient received the treatment at Hull Royal Infirmary on Tuesday afternoon.
It involves inhaling a protein called interferon beta which the body produces when it gets a viral infection.
The hope is it will stimulate the immune system, priming cells to be ready to fight off viruses.
Early findings suggested the treatment cut the odds of a Covid-19 patient in hospital developing severe disease - such as requiring ventilation - by almost 80%.
It was developed at Southampton University Hospital and is being produced by the Southampton-based biotech company, Synairgen.
A course of treatment with the new drug could cost around £2,000, which is not that expensive for a hospital treatment.
"To be viable it will have to represent good value for money," Synairgen's chief executive Richard Marsden said.
Alexandra Constantin, 34, was the first person to receive the treatment as part of this new trial, after she was admitted to the hospital with coronavirus on Monday.
She has a young daughter at home she is desperate to get back to.
Demonstrating the treatment, the nurse handed her a nebuliser that makes the drug into a fine mist, which Alexandra inhaled as deep into her lungs as she could.

How does the treatment work?
Interferon beta is part of the body's first line of defence against viruses, warning it to expect a viral attack.
The coronavirus seems to suppress its production as part of its strategy to evade our immune systems.
The new drug is a special formulation of interferon beta delivered directly to the airways via a nebuliser which makes the protein into an aerosol.
The idea is that a direct dose of the protein in the lungs will trigger a stronger anti-viral response, even in patients whose immune systems are already weak.
Interferon beta is commonly used in the treatment of multiple sclerosis.
Previous clinical trials conducted by Synairgen have shown that it can stimulate an immune response and that patients with asthma and other chronic lung conditions can comfortably tolerate the treatment.

The results of a smaller, phase two clinical trial of the treatment conducted last year were promising.
It suggested the chances of a Covid-19 patient in hospital getting severe disease - requiring ventilation, for example - were reduced by almost 80%.
Patients were two to three times more likely to recover to the point where everyday activities were not compromised by their illness, Synairgen claimed.
It said the trial also indicated "very significant" reductions in breathlessness among patients who received the treatment.
In addition, the average time patients spent in hospital is said to have been reduced by a third, for those receiving the new drug - down from an average of nine days to six days.
But the trial was small, just 100 patients, and more testing is needed before it can be authorised for use.
This new "phase three" trial is much larger. It will involve more than 600 patients in 20 countries.
As in the earlier trial, half the participants will be given the drug, the other half will get what is known as a placebo - an inactive substance.
The team running the trial say they hope it will be completed by early summer.
If the results are anywhere near as good as in the earlier trial, they expect authorisation for the drug to be used in patients in the UK and other countries to follow shortly afterwards.
Synairgen CEO Richard Marsden explains how the treatment uses the protein interferon beta to help fight the virus
"If we had a positive study, we would hope to move rapidly into scaled manufacture and delivery of the drug in clinical practice," said Prof Tom Wilkinson, of the University of Southampton, who is overseeing the trial.
He added that he believes the new drug - if it proves effective - will be a complement to the vaccines that are being rolled out.
He also pointed out it would take a long time for the whole world to be vaccinated. There will need to be treatments for people who miss out on vaccination or choose not to get the jab.
There is also the risk the virus mutates and vaccines become less effective - meaning people begin to develop the disease again.
The treatment is the result of the discovery by a team from Southampton University that people with lung diseases such as asthma and chronic obstructive pulmonary disease (COPD) often had low levels of interferon beta.
"We thought why not boost patients' interferon beta levels by getting them to inhale the protein," Prof Donna Davies, who was part of that team, said.
She said research has now shown that Covid-19 can suppress the interferon beta response. But experts warn that drugs often do not live up to the promise of early trials.
"This is exciting, but we have to see what the phase three results show," says Dr Lamis Latif, a south London GP who has been working in emergency care with Covid-19 patients.
"We've had other drugs in similar circumstances, we've had hydroxychloroquine, for example, but again, when that reached further trials, it wasn't as promising as it initially made up to be.
"So that's something to really take note for this current drug."

VACCINES: When will you be eligible?
LOOK-UP TOOL: How many cases in your area?
GLOBAL SPREAD: How many worldwide cases are there?
THE R NUMBER: What it means and why it matters



Have you been affected by any of the issues raised here? You can get in touch by emailing haveyoursay@bbc.co.uk, external.
Please include a contact number if you are willing to speak to a BBC journalist. You can also get in touch in the following ways:
WhatsApp: +44 7756 165803
Tweet: @BBC_HaveYourSay, external
Please read our terms & conditions and privacy policy
If you are reading this page and can't see the form you will need to visit the mobile version of the BBC website to submit your question or comment or you can email us at HaveYourSay@bbc.co.uk, external. Please include your name, age and location with any submission.
Related topics
- Published15 March 2022
- Published20 July 2020

- Published16 June 2020

- Published26 May 2020

- Published4 May 2020
