Third dose of Covid jab to be trialled in UK
- Published
Matt Hancock: "This will be the first clinical study in the world to look at the impact of a booster jab"
The public is being urged to take part in trials to find out whether a third dose of Covid vaccine could protect against new variants.
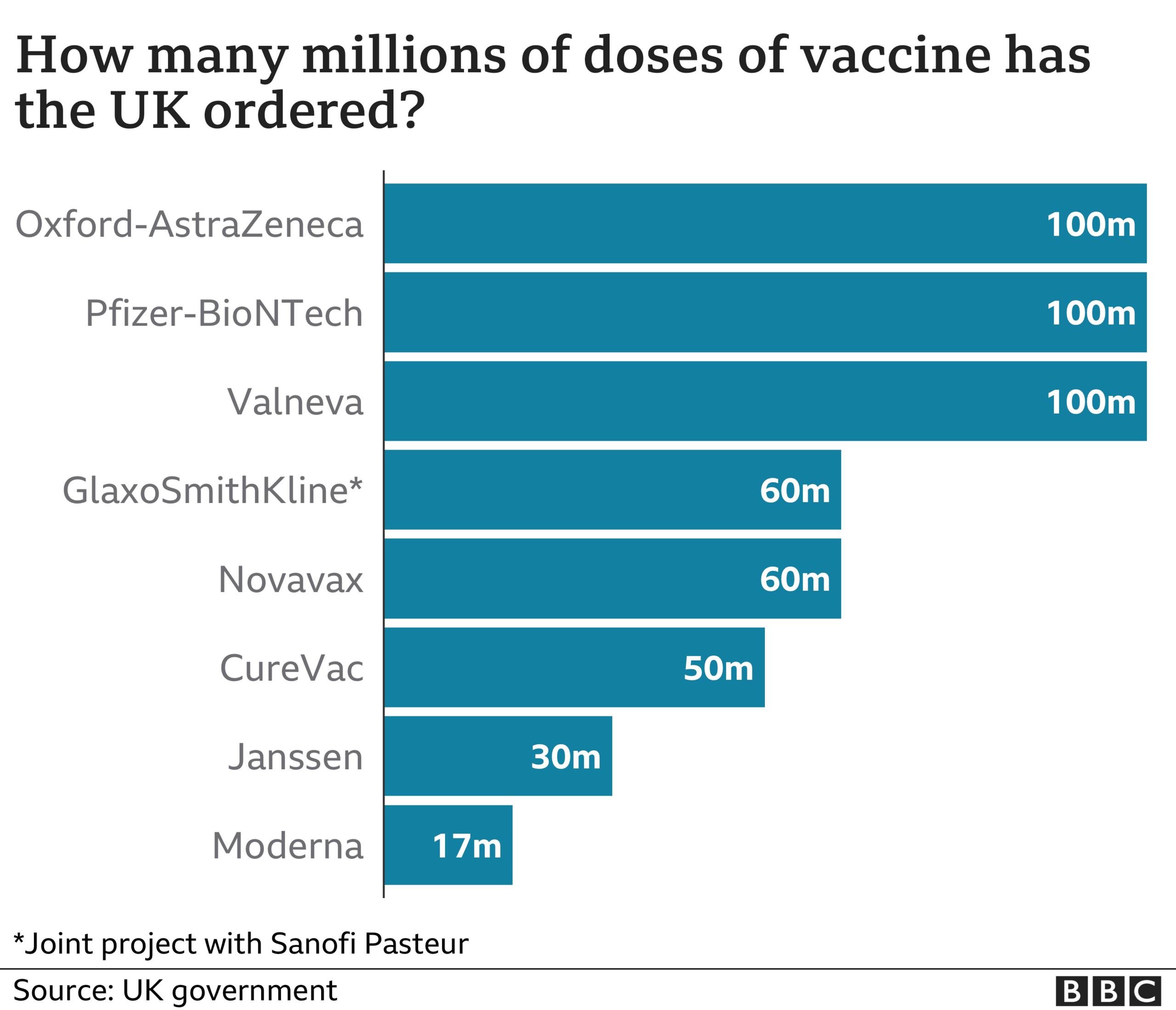
All seven vaccines the UK has ordered will be tested on working-age people and over-75s as part of a randomised trial.
Data on side-effects and immune responses will be gathered.
The findings will help vaccine advisers decide if re-vaccinating some people in the autumn is necessary.
More than 20 million people have been fully vaccinated - with two doses of a Covid vaccine - since the UK's vaccination programme started, but it's still not clear how long protection lasts.
Offering some groups a third dose to boost protection from coronavirus ahead of winter has been suggested - but not confirmed.
Booster campaign
The Cov-Boost study, external, which starts in June, will recruit 3,000 people of all ages who had their first dose in December or January, to test if this is worthwhile.
"It could be that some age groups may not need a booster and others do," said Prof Saul Faust, chief investigator for the trial, from the University of Southampton.
"We are not trying to say one is better than the other.
"The aim is to find out whether there should be a booster campaign and which vaccine to use," he said.
Prof Faust said he was not expecting any vaccines to be "detrimental" but some could cause high fevers or very sore arms, for example, which would be useful to know.
The vaccines will be trialled at 18 sites across the UK, and half doses will also be tested.
The full list of sites is: Southampton, London (University College Hospital, Guy's and St Thomas' Hospital and Northwick Park Harrow), Leicester, Bournemouth, Portsmouth, Wrexham, Bradford, Oxford, Glasgow, Leeds, Cambridge, Birmingham, Brighton, Stockport, Liverpool and Exeter.
Participants will be asked to keep diaries of any side-effects after a third dose, which could be one of seven different Covid vaccines, and researchers will test participants' immune response after one, three and 12 months.
This involves testing their blood for antibodies to coronavirus - high levels are a sign that the body's defences are primed to fight off the virus.
All doses would be from current vaccines, designed to protect against the original form of the virus.
In addition to doses of AstraZeneca, Pfizer and Moderna, some people will be given doses of vaccines from Novavax, Janssen, Valneva and CureVac, which are currently being trialled in large numbers of people, but have not yet been approved by the UK regulator. There will also be a control group, who will be given a dummy vaccine.
Even though the study is using current vaccines, rather than vaccines which have been updated to target new variants, scientists say they expect a boost in antibodies which could be enough to protect against all circulating forms of the virus.
Dr Matthew Snape, associate professor of paediatrics and vaccinology at the University of Oxford, said the study was a world-first and there would be global interest in the results.
Another study, called ComCov, which is testing using different doses of vaccines for the first and second doses found that mixing doses increased mild-to-moderate symptoms after vaccination.
The Cov-Boost study, which costs £19.3 million, is being funded by the government and led by the University Hospital Southampton NHS Foundation Trust, as part of the National Immunisation Schedule Evaluation Consortium (NISEC).
- Published19 May 2021

- Published19 May 2021

- Published13 May 2021
- Published7 June 2021