UK’s most expensive drug Libmeldy saved Teddi Shaw, but is too late for her sister
- Published

Nala (l) and Teddi both have the genetic disorder MLD - but Nala is too old to be treated
A toddler with a rare inherited condition has become the first child to be treated by the NHS with a new life-saving gene therapy.
Teddi Shaw was diagnosed in time because her older sister Nala showed symptoms - but it was too late to treat Nala, who is now terminally ill.
Both girls have MLD, which severely damages the brain and nervous system.
The one-off treatment, called Libmeldy, costs £2.875m and is the most expensive medicine ever approved for the NHS.
The BBC was given exclusive access to follow Teddi's treatment over several months and spoke to other families affected by MLD.

Imagine having two daughters with a devastating genetic condition - but only one can be saved.
Three-year-old Nala - and Teddi, who is 19 months old - both have MLD, metachromatic leukodystrophy.
Children with this fatal genetic disease are born apparently healthy, but MLD gradually attacks the brain and body.
Before Nala became ill, she was a completely normal toddler.
"She was always singing, dancing and spinning around everywhere, always laughing - just a cheeky little girl," says her dad, Jake.
Watch: 19-month-old Teddi is the first child to be treated on the NHS with the new gene therapy
But just over a year ago, Nala's walking gradually became uneven and she started falling over more often. She was also showing signs of a tremor.
Her parents Ally, 32, and Jake, 29, became increasingly concerned. Ally was convinced Nala had a brain tumour.
Initially, doctors reassured them nothing was wrong. But then, in April last year, Jake and Ally took Nala to A&E where she had an MRI scan. Forty-five minutes later they had a likely diagnosis.
"When the doctor said 'It's not a brain tumour,' I was doing cartwheels almost, so excited," Ally says.
But her relief evaporated when the doctor mentioned metachromatic leukodystrophy - which they had never heard of before. When she left the room, Jake Googled the term. "I could tell by his face it wasn't good news," says Ally.
What is metachromatic leukodystrophy (MLD)?
MLD is caused by a faulty gene which means children affected cannot produce an important enzyme called ARSA - a protein that helps the body's metabolism work.
As a result, fatty chemicals called sulfatides build up. These gradually destroy the protective layer around cells in the brain and nervous system, leading to a devastating deterioration. Children lose the ability to walk, talk or eat - and eventually to see or hear.
Because both Ally and Jake are carriers of the faulty gene, they were told Nala's younger sister Teddi had a one-in-four chance of also having MLD.
"I thought to myself, it can't happen again, we can't be that unlucky," says Jake. "When we found out, it was just heart-breaking."

Bittersweet Medicine, external
A cutting-edge treatment turning a terminal diagnosis into hope for a healthy life.
Watch now on BBC iPlayer, external(UK Only)

But for 10-month-old Teddi, there was hope. The disease had not yet affected her and so she became the first patient treated on the NHS with Libmeldy, which must be given before the disease has caused irreparable damage.
Nala's MLD was identified too late for her to be treated. She is already unable to walk or talk, and has to be tube-fed.
"When they told us there was treatment available for Teddi it was kind of a bitter pill to swallow because Nala can't be helped," says Ally.
She says they are sad and "extremely grateful" at the same time.
"I've always said Nala saved Teddi's life. And that's how I wanted to think about it," says Jake.
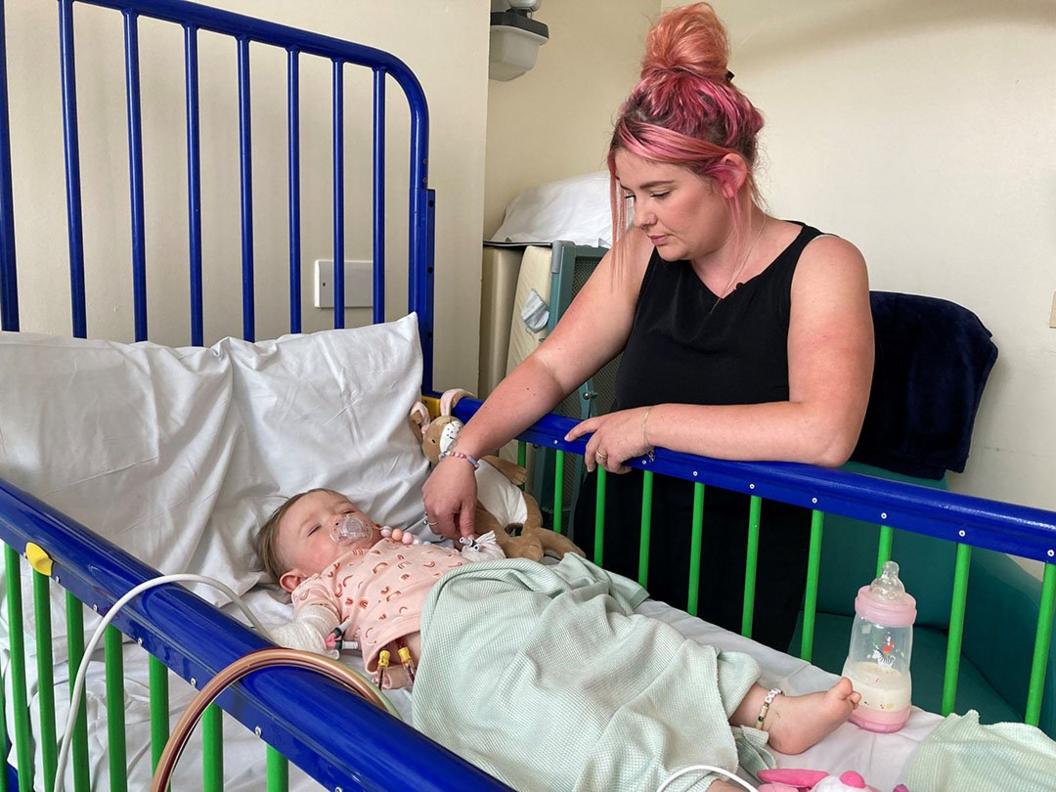
Ally watches while Teddi's stem cells are removed
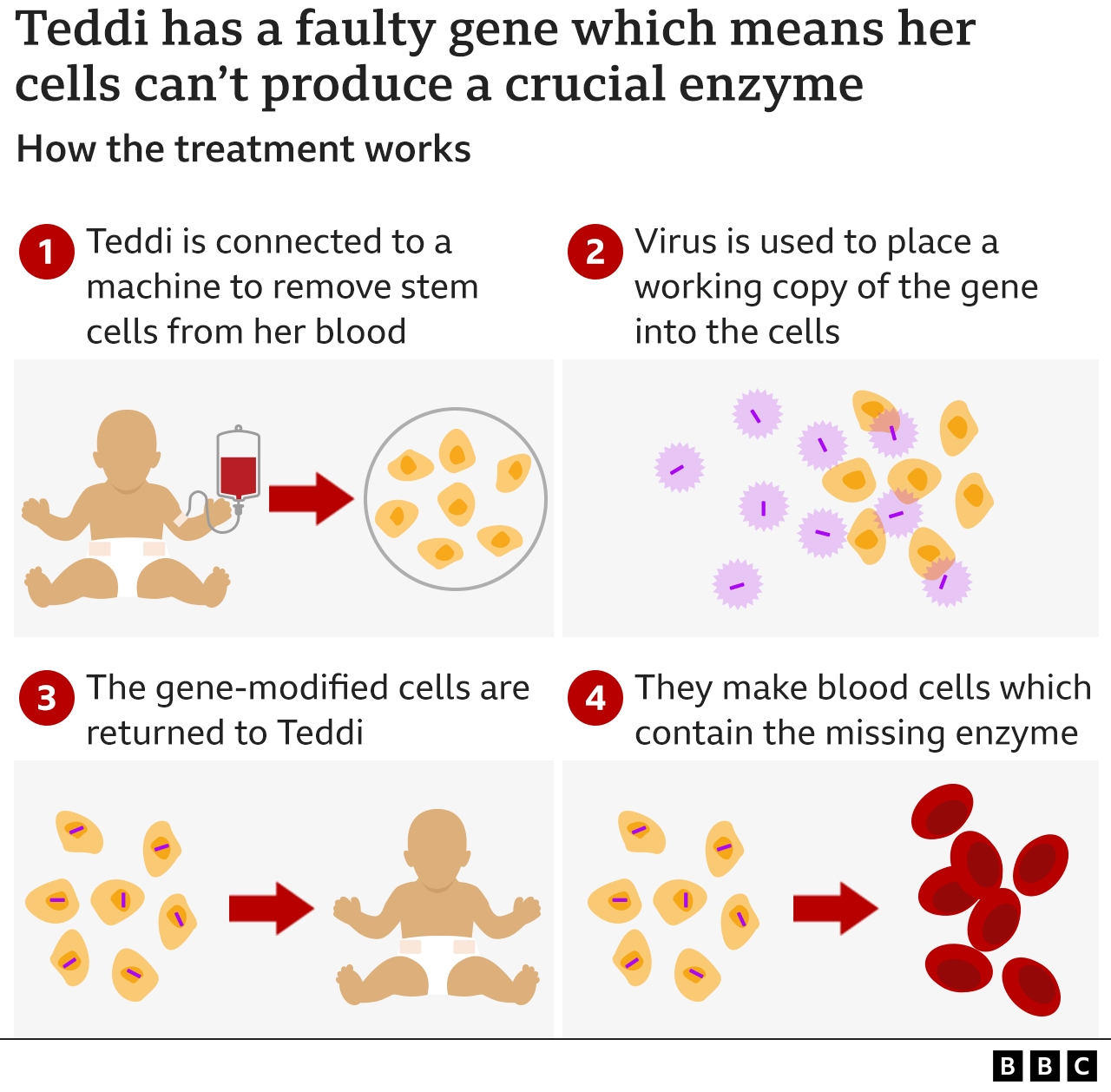
How does Libmeldy work?
Libmeldy involves altering a patient's own cells to correct the faulty gene. In June 2022, Teddi was hooked up to a machine at Royal Manchester Children's Hospital where blood was removed and filtered, so a single bag of stem cells could be collected. The process looks similar to dialysis.
The cells were then sent to Milan, where scientists used a harmless virus to insert a working version of Teddi's faulty gene - the one which should produce her missing enzyme - back into the stem cells. The gene-corrected stem cells were then sent to Manchester to be infused back into Teddi.
Teddi and her mum Ally moved into hospital in Manchester for the duration of the treatment. Ally, formerly a senior staff member in a children's home, has put work on hold. Meanwhile dad Jake, a carpenter, stayed home in Northumberland to look after Nala.
Before she could be given the replacement cells, Teddi had to have chemotherapy to kill off the remaining faulty stem cells in her bone marrow.
In August, we were back in Manchester to watch Teddi receive Libmeldy.
In her hospital room, Teddi, then 14 months old, had chosen that day to attempt her first tentative steps. Mum Ally said her younger daughter was taking it all in her stride.
"She's doing absolutely fine, considering what she's been through," Ally told us. "She's still just her mischievous normal little self."
The infusion of Libmeldy took less than an hour. Over the following days the gene-altered cells migrated to Teddi's bone marrow and began producing the enzyme she had been missing since birth.
What is remarkable is that this is a one-off treatment, with the hope that it provides a permanent fix for MLD.

From BBC Sounds, listen to 5 Minutes On: Nala and Teddi - two sisters, one bittersweet story

Libmeldy was developed by a British company, Orchard Therapeutics. Its CEO and co-founder, Bobby Gaspar, spent many years as a consultant at Great Ormond Street Hospital, while carrying out research into potential therapies.
"Bringing a new medicine to the world that can potentially cure these devastating diseases is incredibly rewarding," he says, adding that it was "a very long journey to develop a medicine like this".
Libmeldy took nearly 20 years to develop, with the first human trials taking place in 2010. It got EU approval in December 2020 and is now available through the NHS.
Doctors who specialise in treating MLD say Libmeldy is a game-changer.
"This truly is a breakthrough," says Prof Simon Jones, one of the consultants involved in Teddi's treatment.
"We have had almost nothing to offer families with this condition for decades. Instead of many years of terrible neurodegenerative disease, we have the potential for a full life, lived healthily."
Now that there is a treatment, it has become even more important to pick up the disease in time.
Teddi's parents, along with other MLD families and the doctors who treat them, are campaigning to have it screened for at birth. In the UK, babies are given a heel-prick blood test which screens for nine genetic conditions, such as cystic fibrosis - but it does not currently include MLD.
"We are letting our children down by not screening for these devastating conditions because they are so preventable if you can identify them at birth," says Dr Gaspar.

The Shaw family - Nala, Jake, Teddi and Ally - at home in Northumberland
Is Libmeldy a cure?
It is too early to tell, but the signs are good. Several children from the UK were involved in clinical trials of Libmeldy in Milan, before it became a licensed treatment.
Joe Elson, who is 12, had his gene therapy in Italy in 2014. Nine years on, he is completely healthy and doing well at school. Watching Joe fly his kite on a beach in Kent, it is hard to imagine that he was born with a devastating disease. It appears that Libmeldy has provided a permanent fix for his MLD.
"It's given him his life back. He makes the most of every moment," says his mum, Nicola.

Joe Elson had gene therapy for MLD in 2014
Joe's MLD was only picked up when his older sister Connie was diagnosed. She died last summer. Nicola told us 13-year-old Connie had lost the ability to walk, talk, eat and hold her head up. She had also lost her vision and hearing, and the ability to smile.
"It's the most horrific, wicked condition that steals these children away," she says.
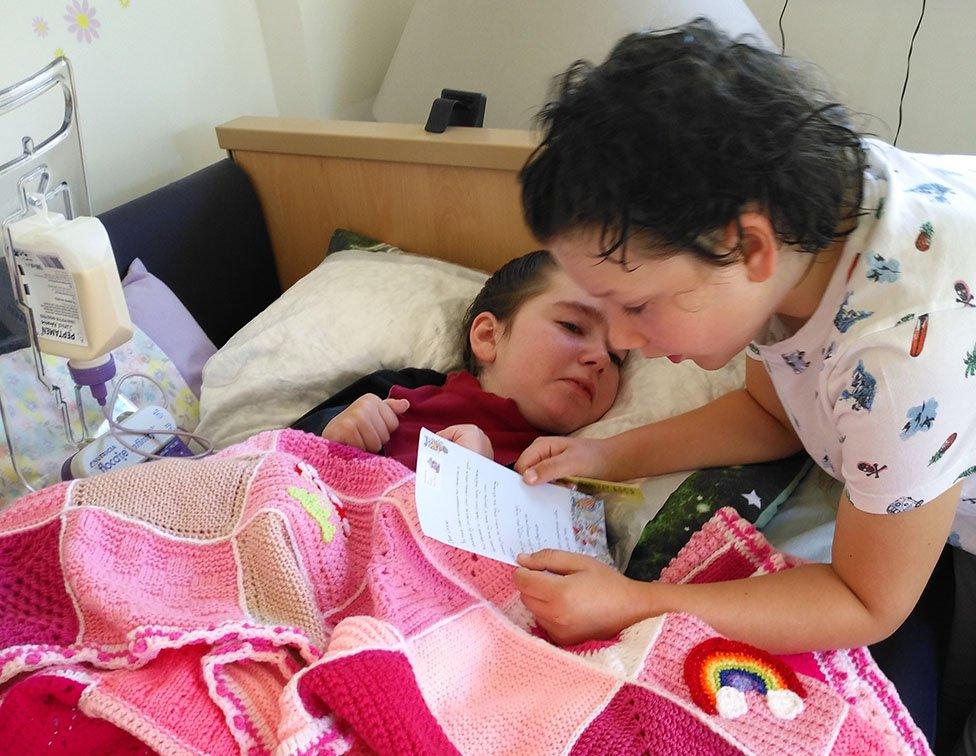
Joe reading to his older sister Connie
It's expected that only about seven or eight children a year in the UK will be eligible for Libmeldy. That is because MLD is rare and usually not diagnosed early enough.
The health assessment body NICE says Libmeldy is one of the most clinically effective medicines it has ever appraised. And, although it has a list price of £2.875m, NHS England has negotiated a confidential discount.
One reason why the price tag is so high is to cover the costs of developing and producing the drug. The price paid by the NHS for this one-off treatment has to be set against the cost of treating children with MLD as they gradually become completely dependent, tube-fed and lose all their senses. And then, there is the suffering endured by patients and their families.
The NHS chief executive Amanda Pritchard describes Libmeldy as a revolutionary treatment offering "a huge moment of hope" for parents and children affected by MLD.
"It means that children like Teddi can do the things that all children should be able to, like going to school and playing with friends," she says.
Back home in Northumberland, Teddi is going from strength to strength.
But seeing her together with her older sister Nala brings home the harsh reality facing Jake and Ally. It is "an absolute blessing", says Jake, that Teddi has received Libmeldy, but "absolutely heartbreaking" to watch Nala's rapid decline.
"Her body is basically kind of gradually shutting down and she will lose most of her senses. So it will come to a point where there's nothing left for her to lose," Jake says.
"You feel like you're grieving from the very start because your child is disappearing almost in front of your eyes," says Ally.
The Shaws know that if Nala had been diagnosed earlier she might have been treated, rather than facing a terminal illness.
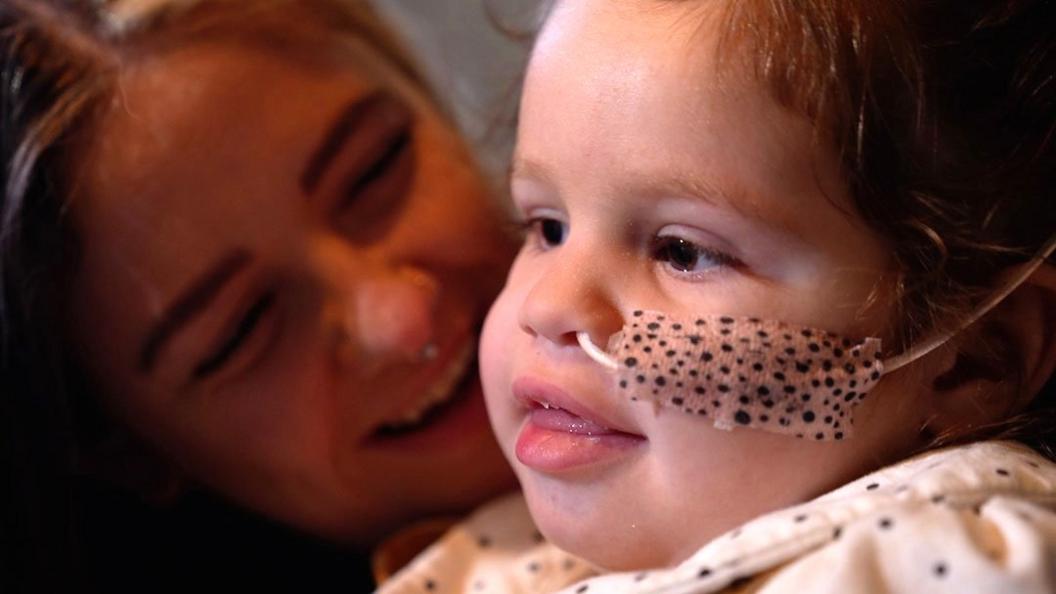
Nala now has to be tube-fed
Jake, who plays guitar, has recorded a song for his daughter entitled "Lay You Down Easy, external", which he hopes will raise awareness of the disease, with any money raised going to the MLD Support Association.
Although MLD is not currently screened for at birth in the UK, small pilot studies to screen newborns have begun in five countries - including Germany, where testing has identified the first patient with the condition.
Later this year, Genomics England will start a pilot project offering whole genome sequencing to 100,000 newborns. This will screen for about 200 treatable conditions, and may include MLD.
Could other rare diseases be treated this way?
The simple answer is yes. Royal Manchester Children's Hospital is trialling two other gene therapies for rare disorders, Sanfilippo and Hunter syndromes.
The director of the Paediatric Bone Marrow Transplant Programme, Prof Rob Wynn, says many of his young transplant patients have genetic diseases, and he thinks the approach of correcting the conditions using gene-modified stem cells will be "transformative".
Nala's parents say it would be a fitting testament to her if newborn screening for MLD became the norm.
"I would like to think that if another child was born with MLD, it could be picked up quick enough for them to be saved," says Ally.
Additional reporting by Nicki Stiastny
Follow Fergus on Twitter, external
Related topics
- Published10 June 2019
- Published4 February 2022
