Thanks for joining uspublished at 15:31 GMT 30 November 2022
We're bringing our Q&A on a breakthrough drug to treat Alzheimer's to an end shortly - thanks for sending us your questions.
Thanks also to our health and science correspondent James Gallagher for providing plenty of really useful answers.
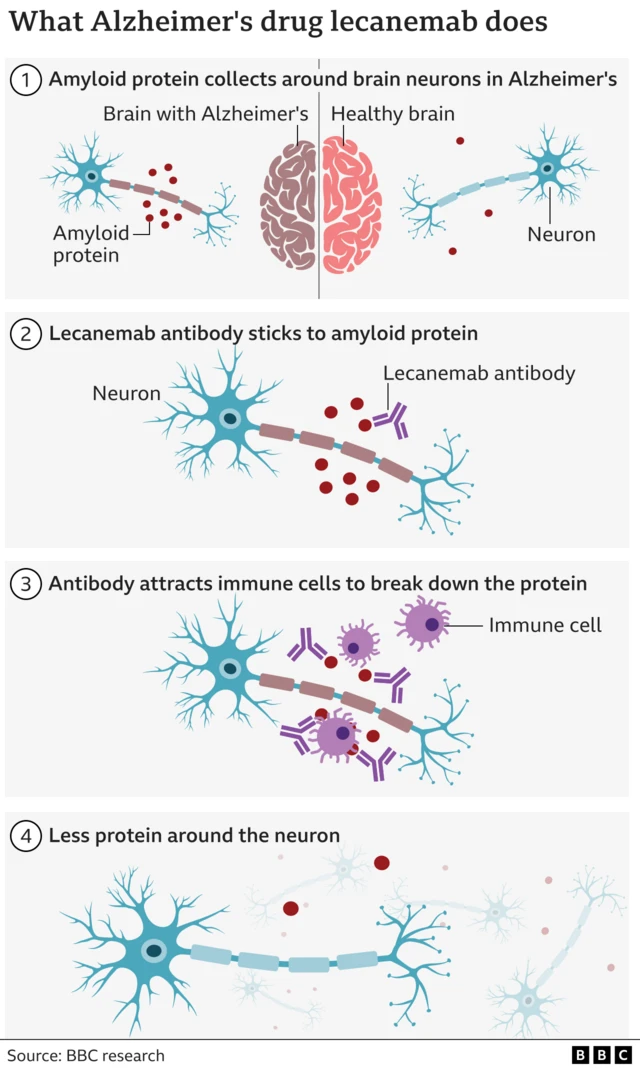
And if you want to know more, read on about on lecanemab, the first drug to slow the destruction of the brain in Alzheimer's, here.
Today's page was also written by Marita Moloney, Gem O'Reilly and Sam Hancock, and edited by Dulcie Lee and Andrew Humphrey.