Sodium: Getting rid of dirt - and murder victims
- Published

Be warned, this article contains material some readers may find distressing. That's because it's about sodium - and the story of one of Italy's most notorious serial killers illustrates elegantly some of this element's unique properties.
Sodium is highly reactive, which means the soft, silver-coloured metal is never found in its pure form in nature. Cut a piece of pure sodium - you can slice it with a knife - and it will cloud and tarnish before your eyes as it reacts with the air.
This reactivity, and its relative cheapness, are what make sodium so useful to industry - and also to murderers.
You probably know the element from its most commonplace form, table salt, or sodium chloride. But the great feedstock of the chemicals industry is a different sodium chemical - sodium hydroxide, better known as caustic soda.
It is produced from salt by electrolysis.
An electric current acts as what Prof Andrea Sella of University College London describes as "a pair of chemical shears", snapping the tight bonds that hold the sodium and chlorine atoms together.
Caustic soda is extremely corrosive.
These days its main use in industry is to break down a particularly robust form of organic matter - wood. The process invented by German chemist Carl Dahl in the 1880s turns wood into pulp using caustic soda and sodium sulphate. It was dubbed the "Kraft" process, from the German word for "power", because of the strength of the paper that results from it.
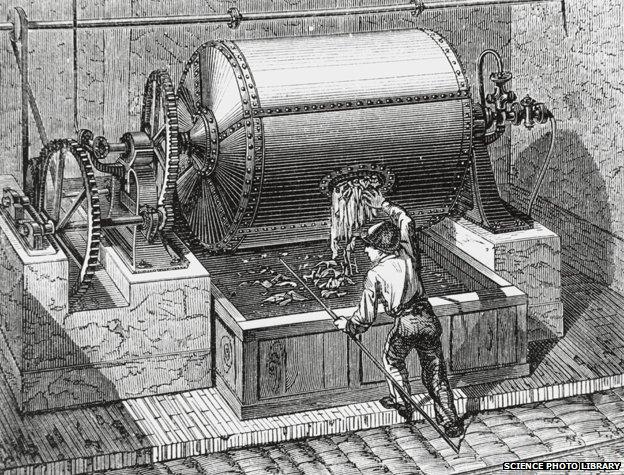
Today, over three-quarters of pulp production uses this technique.
Caustic soda's aggressive action is also exploited to refine bauxite ore into aluminium and to remove impurities from oil, and it has all sorts of uses much closer to home. You probably use it to unblock your drains or, together with sodium hypochlorite, as bleach.
It turns up in these products because caustic soda is very effective at digesting grease and fat - the same property that has attracted serial killers such as Leonarda Cianciulli.
Born in 1894 in Montella, in southern Italy, by the 1930s she was married and running a small shop in the northern town of Correggio. A popular woman, friends and neighbours thought of her as a kind woman and a doting mother to her four children.
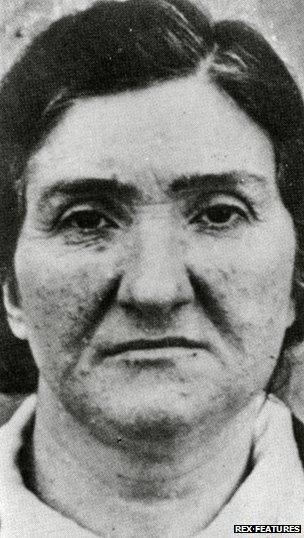
But she was also very superstitious. The story goes that she was warned by a gypsy fortune-teller that her children were at risk, and became convinced the only way to protect them was with a human sacrifice.
Between 1939 and 1940 she lured three middle-aged women into her little shop.
She drugged them, killed them with an axe, and then disposed of their bodies by boiling them in caustic soda.
In her memoir she described the "thick dark mush" the process produced.
Others have followed her gruesome example.
In 2009, the Mexican Santiago Meza, dubbed the "stew maker", confessed to having been paid by a local drugs gang for several years to dispose of the corpses of 300 unfortunate members of a rival gang. He too used caustic soda.
What makes Mrs Cianciulli so exceptional, however, is that she went on to exploit another of sodium's special properties. She used it to turn at least one of the murdered women into soap.
"She ended up in the pot, like the other two," the killer wrote in her memoirs, referring to her last victim.
"Her flesh was fat and white. When it had melted, I added a bottle of cologne, and after a long time on the boil I was able to make some most acceptable creamy soap. I gave bars to neighbours and acquaintances.
"That woman was really sweet."
Leonarda Cianciulli earned the nickname la Saponificatrice di Correggio, the soap-maker of Correggio.
What is it that makes soaps soapy? It's the fact that "they allow us to mix oil and water", says Prof Sella.
"The soap molecule looks a little bit like a tadpole. You have a head and a long wiggly tail."
This molecular "tadpole" combines two contradictory properties. The sodium-containing head is powerfully hydrophilic (attracted to water). The fatty acid tail is hydrophobic (repelled by water).
If you have a greasy stain on a T-shirt and soak it in soapy water, then the tails of the soap molecules are repelled by the water and drawn to the grease.
This works to loosen the grease and break it up into microscopic globules suspended in the water, each surrounded by soap molecules with their hydrophilic sodium heads pointed outwards.
It makes soap a very effective surface active agent or "surfactant", providing a bridge between materials that wouldn't otherwise mix.
The most familiar use of soap is to wash our skin and hair, but sodium-based surfactants are also used to mix products like paints and cosmetics.
Now, if you are glad that I have washed away the unpleasant topic of murder with the far cleaner one of soap, I have bad news for you. Sodium is a major killer in its own right, in an entirely different context - food.
The element has plenty of bizarre niche uses in the food industry. It is used to put the crust on pretzels, to cure fish and to soften olives. It is also the reason why fizzy drinks are often called "soda".
In the 19th Century, soda water was often manufactured by dissolving sodium bicarbonate - the same stuff you might use in baking - in water. The sodium wasn't actually that important, it was the bicarbonate part that put the bubbles in your bottle.
These days most drinks are carbonated by simply pumping carbon dioxide into the liquid under pressure, dispensing with the sodium altogether. Nonetheless, the name "soda" has stuck.

But when it comes to sodium in food, sodium bicarbonate and caustic soda are small fry. The biggest source is the chemical I began this article with - sodium chloride, or table salt.
Small amounts of sodium are vital for the body. Sodium ions are an essential part of our brain chemistry, and sodium's hydrophilia - which makes it so great in soap - is exploited by our bodies to regulate blood pressure.
A single sodium ion can attract up to 25 water molecules, so the more sodium there is in our bloodstream, the greater the volume of fluid that can swell our veins.
So salt is good for us in limited quantities. But prehistoric humans presumably found it hard to get the salt they needed in their diet, because we have evolved a pronounced taste for the stuff.
You've probably heard that the word salary comes from Latin, salarium.
But this isn't because Roman legionaries were paid in wafers of salt, as is often claimed. Classical scholar Armand D'Angour says the word salarium actually means "salt money", in the same way that these days we talk about "beer money".
They used some of their income to buy salt - then a valuable commodity - to season their food.
Of course these days salt is cheap and plentiful, and that is the problem. It is all too easy to eat too much - or, at least, that is the consensus among the world's governments and health authorities.
"Blood pressure is the biggest single cause of death in the world, through 60% of strokes, 50% of all heart disease," says cardiology professor Graham MacGregor of Queen Mary College London, a leading campaigner for lowering salt consumption.
"Anything that puts up our blood pressure is very dangerous, and the evidence is that salt's the main factor."
That evidence includes a study recently published by MacGregor claiming that a government campaign in the UK, which cut average salt intake from 9.5g to 8.1g per day, is preventing about 9,000 deaths a year.
But there are some who dispute the evidence. Morton Satin, is one. He is the spokesman for the Salt Institute, which represents the US salt industry.
The human body is very efficient at controlling blood sodium levels, Satin argues, and people's preferences are well attuned to the correct level of salt intake.
While he agrees that some people do need to cut their salt consumption, too little salt can also be dangerous for some people, he says.
Arguments like these have halted moves in the US to get food manufacturers to cut the amount of salt they use.
But MacGregor will have none of it.
"You work for a public relations company that's trying to stop any reduction in salt intake," he says, going on the attack.
"You are doing your best to fight your corner, just like the tobacco industry."
MacGregor suggests we take Satin's claims with a pinch of sodium chloride.
Follow @BBCNewsMagazine, external on Twitter and on Facebook, external