Plutonium: The scary element that helps probe space's secrets
- Published

Plutonium may be the most feared and fearsome substance in the entire periodic table.
It's best known as the main ingredient of atomic bombs like the infamous Fat Man, dropped on Nagasaki on 9 August 1945, which killed some 70,000 people. Japan surrendered six days later, but the threat of nuclear annihilation locked the world into Cold War for decades.
Yet the story of plutonium is not all about Armageddon or the threat of it. It is also the story of an incredible voyage of discovery into an unknown world.
You've probably heard the quote "Houston, we've had a problem." It was what Commander Jim Lovell told the Nasa command centre back on Earth in the moments after the Apollo 13 spacecraft had been rocked by an explosion.
It was April 1970, and Apollo 13 was 56 hours and 200,000 miles into its mission, mankind's third attempt to land people on the moon.
One of the oxygen tanks had exploded. The astronauts were forced to turn off the power supply, which caused the temperature on board to plummet and carbon dioxide levels to rise.
Lovell and his crew had to retreat to the lunar module, which carried a suite of scientific instruments powered by a battery containing 8.5lb of pure plutonium.
Had things gone to plan, the battery would have enabled the instruments to gather information on the lunar surface for decades.

Apollo 13 Commander Jim Lovell carrying a plutonium battery and scientific equipment during training
Plutonium has since been key to a number of more successful missions. The Voyager space probes contain batteries that still provide an estimated 300 watts of power today, down from 500 watts when they were launched in 1977. The Mars rover also relies on plutonium's heat to stop its joints freezing, as well as for power.
The battery works because plutonium's nucleus is far bigger than any naturally-occurring element, and that makes it unstable. It cracks open producing radiation, and also heat, which can be converted into electricity.

Plutonium: Key facts

Named after Pluto, when it was considered the final planet in the solar system
Radioactive element of the actinide series in the periodic table, with atomic number 94
Only trace amounts exist naturally on Earth
Silvery metal that takes on a yellow tarnish in air
The heat generated by decay in relatively pure plutonium-238 is such that a solid sphere the size of a golf ball will glow red

The plutonium in these batteries isn't the same as the stuff that atomic weapons are made of, plutonium-239 - which is perhaps just as well, given that the Apollo battery ended up with the rest of the lunar module, deep in the waters of the Tonga Trench.
Plutonium batteries use a different isotope, plutonium-238, which contains one neutron fewer in its nucleus, and decays quite quickly. It has a half-life of 88 years, a fraction of the 24,000-year half-life of plutonium-239, or the 80-million-year half-life of plutonium-244.
But even 80 million years is peanuts compared with the 4.5 billion years our planet has existed, which is why only minute traces of plutonium-244 remained on Earth - until 1940.
This was when another great voyage of discovery began, this time into the unknown chemical world of "trans-uranic" elements.
"Uranium for a long time was seen as the end of the periodic table, Ultima Thule," explains Prof Andrea Sella of University College London, employing a term medieval geographers applied to a place beyond the borders of the known world. "It was as far as you could go."
That began to change in 1932, with the invention by the American scientist Ernest Lawrence of the cyclotron - a device for accelerating particles around a circular chamber using electromagnets.
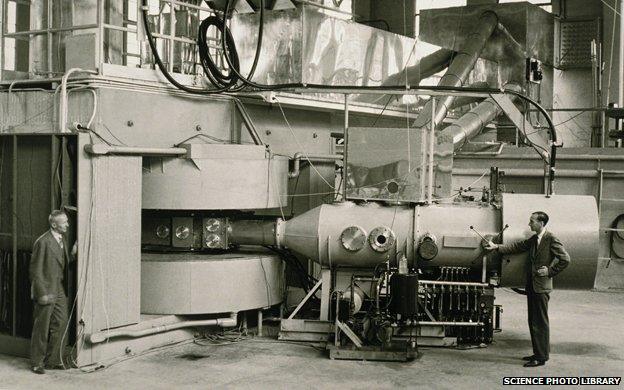
The 60-inch cyclotron built by Ernest Lawrence at the Lawrence Berkeley Laboratory in California
By smashing atoms and particles together, it achieved nothing less than alchemy, transforming one element into another. What makes an element chemically unique is the number of protons in its nucleus. Force another proton in, and suddenly you have a whole new chemical. This is how synthetic plutonium came to be created in December 1940.
A team led by radiochemist Glenn Seaborg used the cyclotron to bombard a sample of uranium with deuterium, creating an element that had first been identified earlier in the year by Seaborg's colleague Edwin McMillan - neptunium, as we now call it.
It decayed in two days, yielding another new element - plutonium.

Glenn Seaborg in 1946, five years before he won the Nobel prize
Berkeley seems an unlikely place for this sinister substance to be created. The warm Californian sun filters through the eucalyptus trees on to the laboratory buildings that hug the hills above San Francisco bay.
But in 1940 much of the world was at war and the race was on to create the most lethal weapons ever seen - atomic bombs.
Now, there are naming rights associated with the discovery of a new element and fortuitously both Seaborg and a UK team, which had come across it simultaneously as a by-product of a nuclear reaction, proposed the same name.
Uranium, element 92, is named after the planet Uranus. The next planet out is Neptune, hence element 93 became neptunium - and logically element 94 became plutonium, after what was then believed to be the final planet in the solar system.
Prof Seaborg playfully suggested that this be abbreviated to Pu - "poo". It passed without comment into the periodic table and plutonium remains Pu to this day.
Plutonium-239 atoms fire out neutrons as they decay. Put enough close enough together and you get an explosive chain reaction.
I was lucky enough to meet the famous nuclear scientist Heino Nitsche at Berkeley just a few days before he died on 15 July 2014, external.
No fan of nuclear weapons, he compared plutonium to a boxful of mousetraps loaded with ping-pong balls, into which you drop one ball to detonate it.
The development of the atomic bomb was not the only research into plutonium and its kin that raised ethical questions.
In the 1990s, an American journalist named Eileen Welsome of the Albuquerque Journal won the Pulitzer Prize after she brought to light secret studies commissioned by the US military, external into the effects of radiation exposure on the human body.
These included experiments conducted at the behest of the US military on human subjects without their consent. Prisoners and hospital patients were used as human guinea pigs. In one experiment doses of radiation were even fed to orphaned children with their breakfast.
The investigative journalist Peter Marshall reported for the BBC on these experiments back in 1994.
In 1994, former pupils at an orphanage talk to Peter Marshall
In one instance, he says, a plume of radiation 700 times the level regarded as safe at the time, was released above the Hanford nuclear plant in Washington State.
The experiments were carried out at the darkest period of the Cold War, Marshall explains, when the American authorities were terrified about the prospect of a nuclear war.
He managed to secure an interview with a then venerable Glenn Seaborg, who had been head of the Atomic Energy Commission that co-ordinated this radiation research.
Seaborg told him that he didn't believe that anyone in the Commission was responsible for the experiments.
"If a hospital or a university wants to carry on an experiment then in the US, in the land of the free, then they carry out that experiment," he said. "There is no-one in Washington that is directing you to do or not to do a certain type of experiment."
Glenn Seaborg in 1994 says scientists were looking for a way of removing plutonium from the body
As Peter Marshall points out, even back in the 1950s many in the scientific community were worried about the ethics of these experiments - the leading radiation biologist Joseph Hamilton compared them to Nazi experiments, saying they had "a little of the Buchenwald touch".
But while the discovery of plutonium had military researchers probing the effects of radiation, it also opened the door to a whole new field of research that enabled fundamental physicists to revolutionise chemistry.
Three research centres - Berkeley in California, Dubna in Russia and Darmstadt in Germany - raced to see just how many more synthetic elements could be created using the descendants of those early cyclotrons.
A total of 24 new elements have been confirmed to date and two more are pending confirmation, so the periodic table now includes up to 118 elements rather than just the original 92.
The names of these new elements reflect the preoccupations of the people who discovered them.
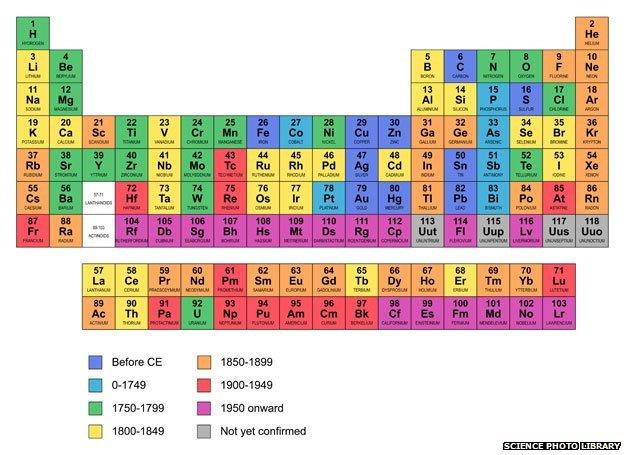
The actinides form the bottom row... the transactinides are those numbered 104 and above
Naturally there are berkelium, dubnium and darmstadtium, as well as livermorium - named after the Lawrence Livermore National Laboratory that, among other things, ensures that the US nuclear stockpile does not decay too quickly.
Many are named after great scientists: einsteinium, curium, fermium, mendelevium, bohrium and rutherfordium.
Others, such as americium, californium and hassium (named after the home state of Darmstadt), have patriotic roots.
Glenn Seaborg was immortalised in his lifetime by element 106, seaborgium, which he considered a far greater honour than the Nobel Prize he won along with McMillan in 1951.
It was well earned, says Prof Nitsche, for Seaborg's impact on the periodic table went much further than just seaborgium or Pu.
His great insight was that many of these new man-made elements formed a completely new series of the periodic table, which he called the actinides, analogous to the lanthinide series that contains the so-called rare earths.
Seaborg's colleagues counselled him to keep his heretical views quiet, lest he destroy his reputation. But Seaborg refused, and was vindicated.
Many of the trans-uranic elements are quite ephemeral. Take element 114, flerovium - named after the founder of the Dubna research centre - the existence of which was confirmed by a team under Nitsche's leadership. It lasts all of two-and-a-half seconds before decaying into oblivion, he told me with a cheerful laugh.

The late Professor Heino Nitsche
Yet many have found useful, indeed life-saving, applications for these elements besides the fuel for atomic batteries.
Take a look around you, there may well be a smoke alarm within a few metres of where you are sitting. Glance at the back of it and you'll see a little version of the yellow trefoil radioactive hazard sign and the letters "Am241".
Smoke detectors work thanks to a tiny trace of americium, the next element after plutonium.
They contain a sensor that detects the radiation, and if smoke blocks the flow of particles to the sensor the alarm goes off. It's as simple as that.
(Please now return the smoke alarm to wherever you got it!)
Scientists are also devising ingenious new ways to use radioactive elements to help cure cancer.
They are using antibodies and other molecules to deliver individual nuclides directly to cancerous cells. The idea is that the radiation they emit destroys the cells but doesn't penetrate any further into the body and damage other organs.

Apparently this is especially effective in late-term chemotherapy and can sometimes give people several years of extra life.
Smoke detectors and cancer treatments - these are the fruit of a research effort that originally aimed to create the world's most life-destroying weapon.
As Prof Sella notes: "The interesting thing is the way really useful applications come out of sometimes really quite questionable research."
Correction: 21 September 2014: An earlier version of this story incorrectly stated that the plutonium battery on Apollo 13 helped save the astronauts' lives.
Subscribe to the BBC News Magazine's email newsletter to get articles sent to your inbox.