Covid-19: How the vaccine will reach your arm
An unprecedented global scientific effort has led to the development of a number of coronavirus vaccines, which promise to help protect the world's most vulnerable from the devastating disease Covid-19.
Scroll down to find out how those vaccines got from the science lab to people's arms in record time.
Vaccines begin life in the lab
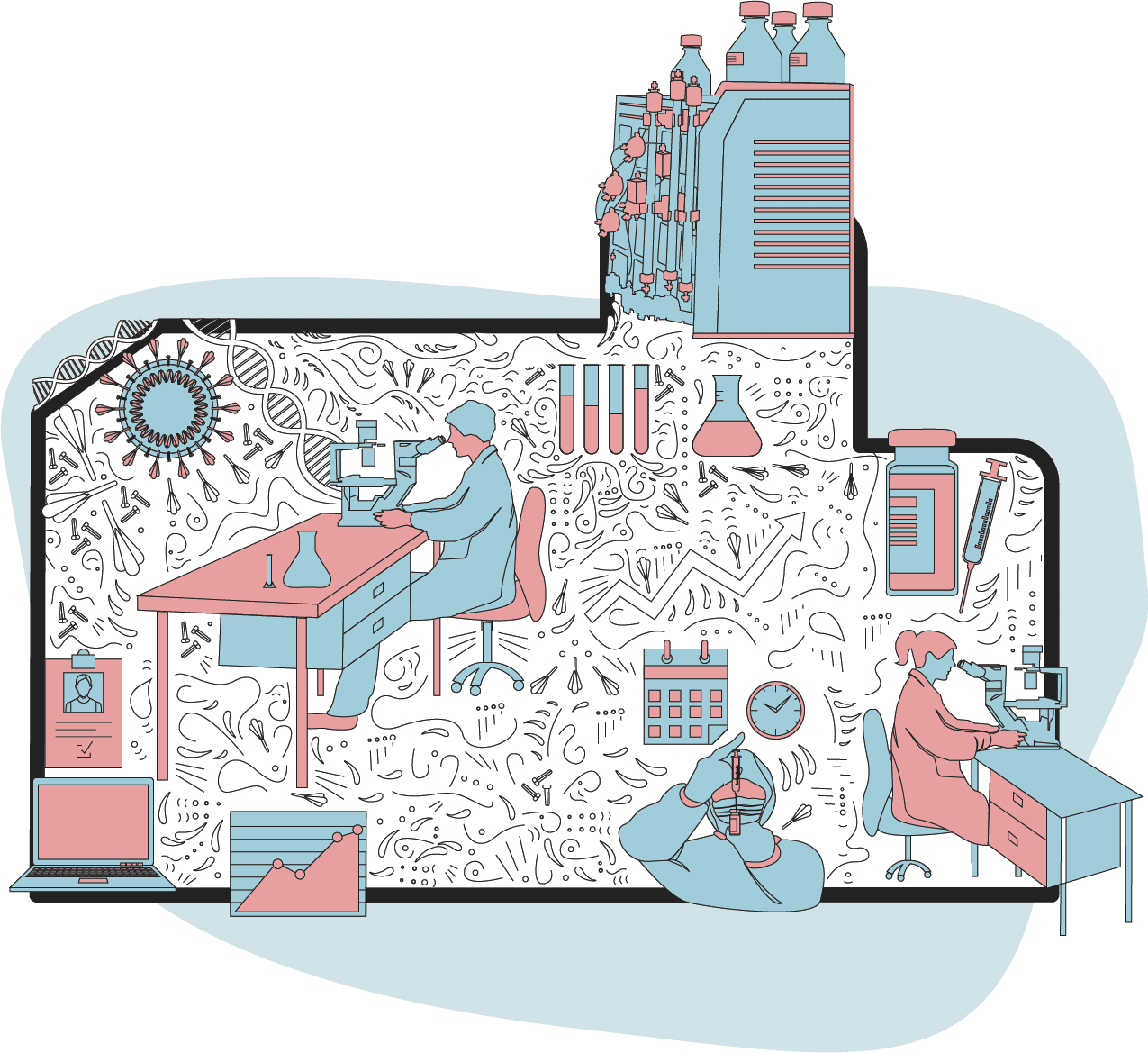
Scientists began the quest to find a vaccine against the new coronavirus when its genetic sequence was released in January 2020.
Collaborating like never before, teams across the world worked on multiple stages of development at the same time - compressing 10 years' work into less than 12 months.
Many had studied other recent coronaviruses that had caused Sars and Mers - so had a head start.
Researchers studied the virus in detail to identify an antigen - a tiny part of it that would trigger our body's immune response.
Most of the successful jabs contain tiny, harmless fragments of the virus or blueprints for making those fragments inside our bodies.
Researchers tested these antigens using computer models and cells in the lab, monitoring side-effects.
The vaccines are then tested on humans
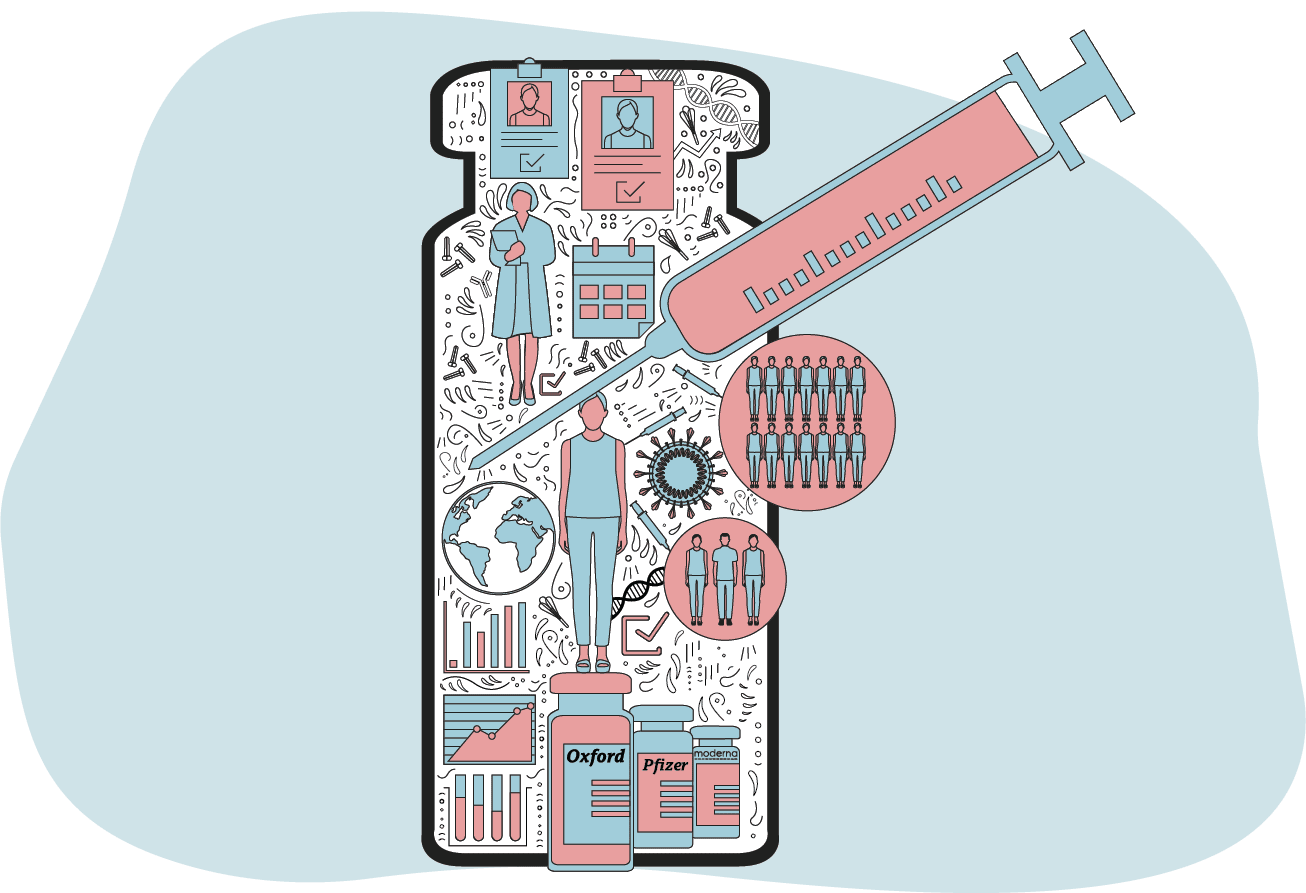
Once these lab-based trials were completed, the vaccines were then given to volunteers across the world to ensure they were safe and effective but also to calculate dosage.
While it usually takes up to 10 years to conduct these trials, for Covid-19 the phases ran simultaneously to speed things up.
The results of the successful trials were then sent for approval to bodies that regulate the safe use of medicines.
These organisations and their scientists critically assessed each vaccine's safety, quality and effectiveness to decide whether to give it the green light.
To speed up approval, the UK regulator used a system called "rolling review". When enough data was available from ongoing trials it was assessed immediately, rather than in one dump at the end.
Doses are mass produced at a pharmaceutical plant
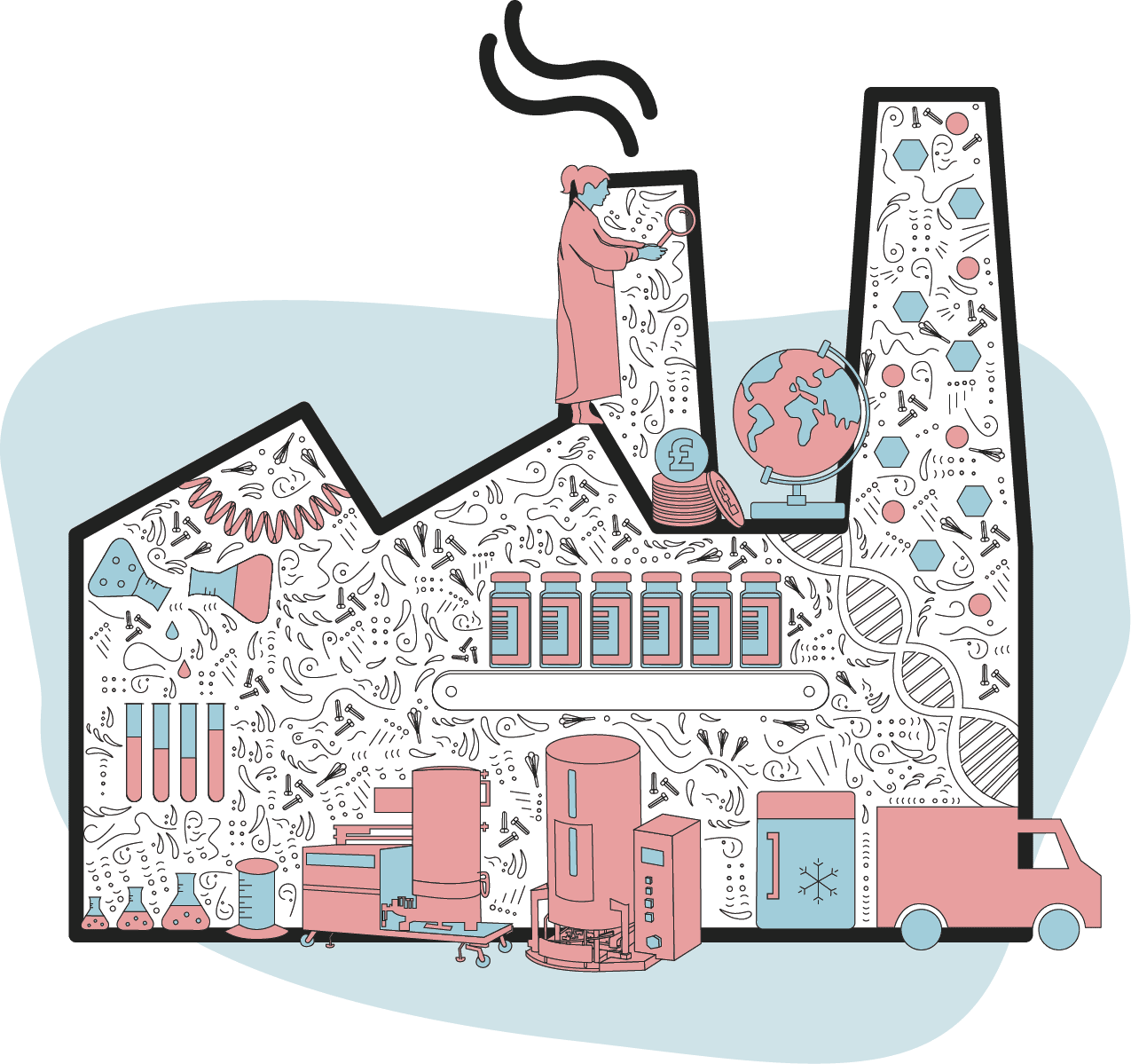
Usually it is at this point - once a medicine has been approved - that pharmaceutical companies begin to scale up production.
But with Covid vaccines, manufacturing capacity was built up much earlier - while research and development was still in progress - because of the large amounts of cash backing the quest.
This meant when safe and effective vaccines were found and approved, companies were ready to distribute them as quickly as possible.
The process of making a vaccine involves producing the active ingredient in large quantities and mixing it with other ingredients, such as stabilisers.
Something that improves the immune response - known as an "adjuvant" - is also often included.
These large batches of vaccine are checked for quality before being put into to vials in a sterile production plant and shipped.
Vaccines are transported in cold chains
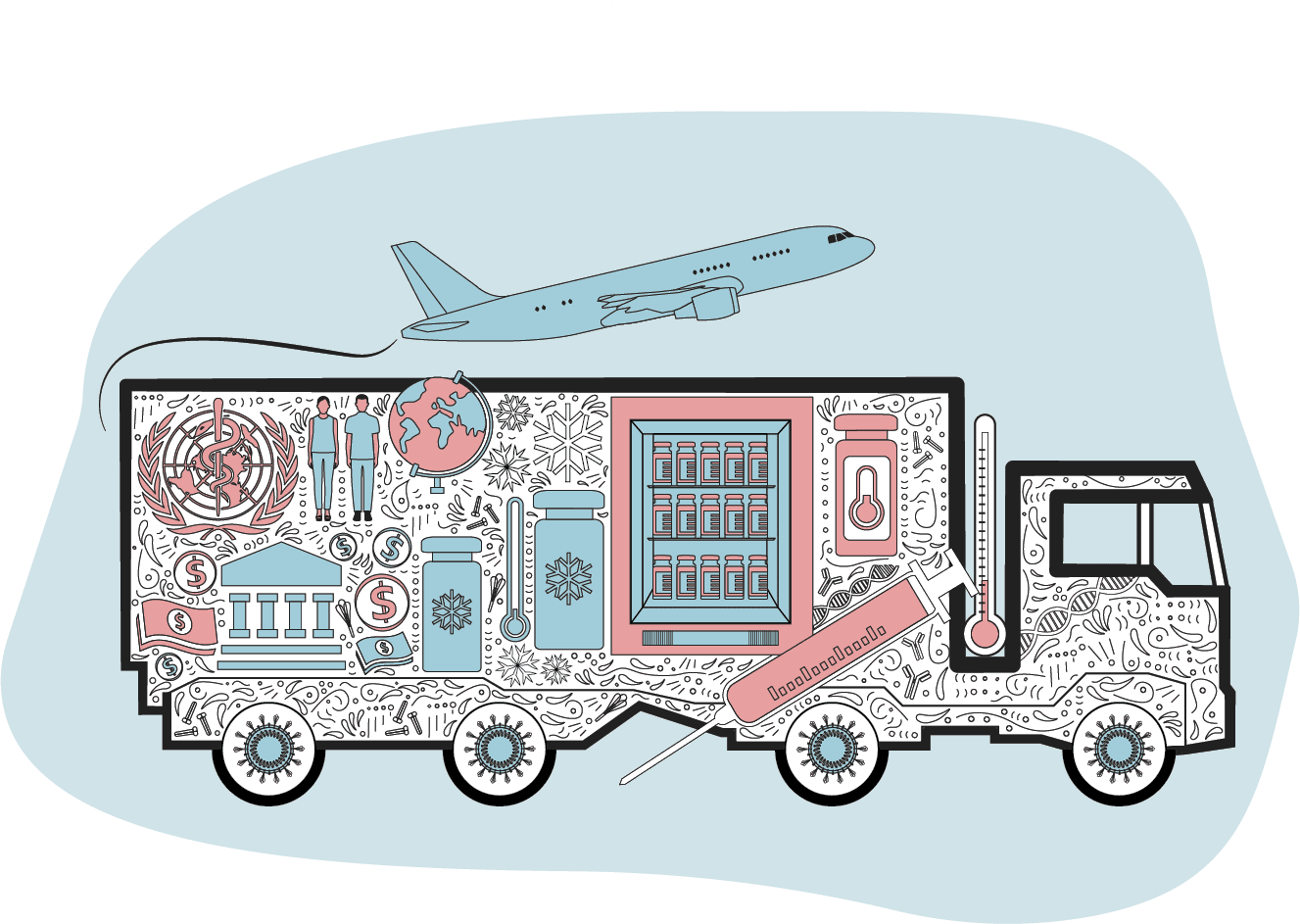
Once released by manufacturers, vaccines are shipped via a "cold chain" to ensure they are always kept at the right temperature.
Most traditional vaccines need to be kept at between 2C and 8C, but some of the Covid-19 jabs require it to be much colder.
The Pfizer-BioNTech vaccine, for example, needs to be stored at a very cold -70C.
However, the British-made Oxford-AstraZenca vaccine can be kept at normal fridge temperatures, so faces fewer challenges and can use established cold chains used for other drugs.
Making sure that the vaccines are available to those who need them most, not just those who can afford to buy them, is crucial to ending the global pandemic.
Governments in high-income countries have placed massive orders for multiple vaccines, but the World Health Organization has set up a scheme - the Covax programme - to ensure vulnerable people in low and middle income countries also receive supplies.
Doses are taken from central hubs to vaccine centres
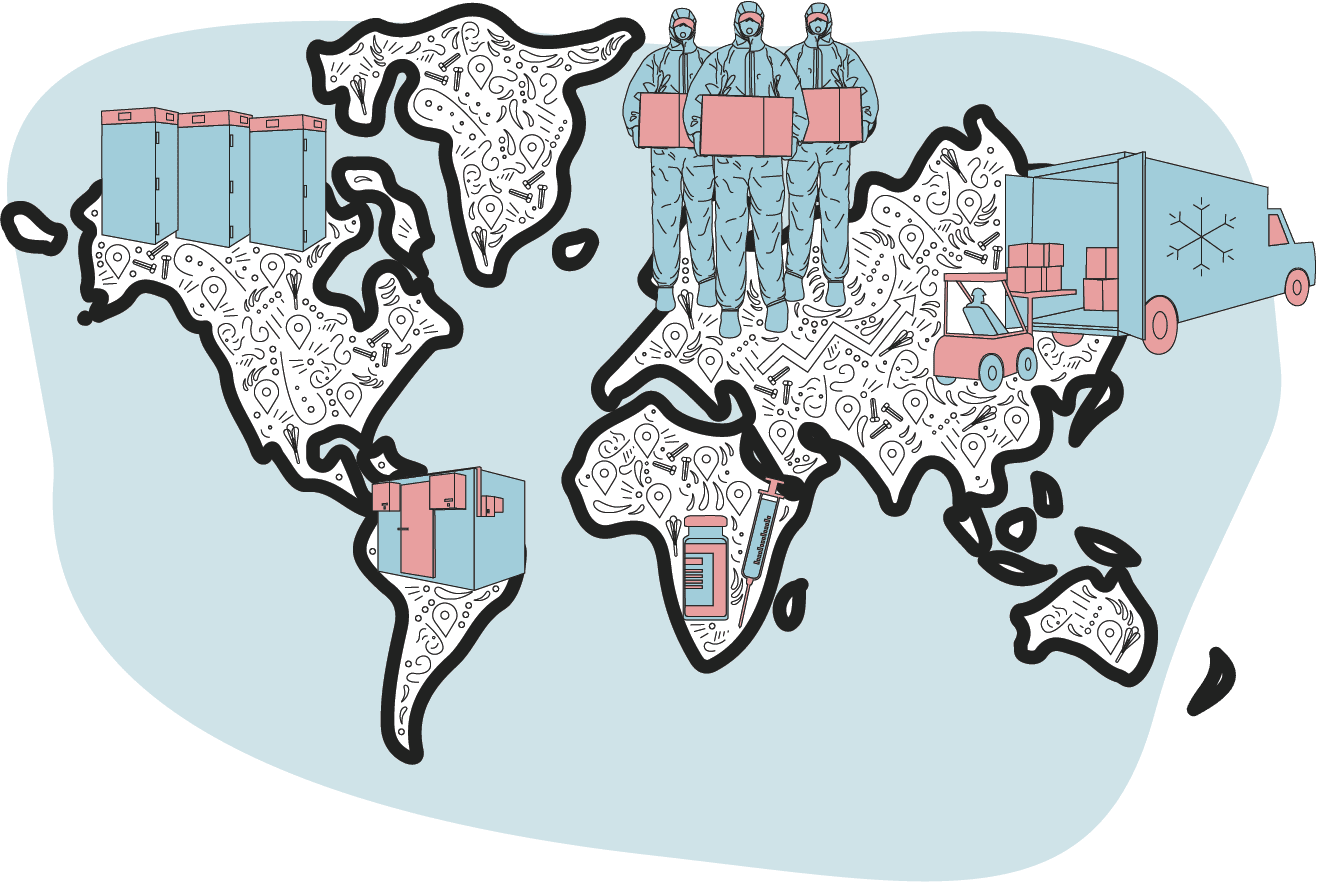
Once a vaccine reaches a destination country it is batch-tested for quality at a secure site before being distributed.
The Pfizer vaccine requires specialist freezers and a number of countries have set up freezer farms - depots containing large numbers of deep freezers - to keep it safe.
Once cleared by testers, smaller batches are transported again in temperature-controlled vehicles to hospitals, pharmacies, clinics and other vaccination teams.
Supplies may also make another pit stop at regional storage sites before heading to clinics.
Vaccine centres deliver the vaccine to patients
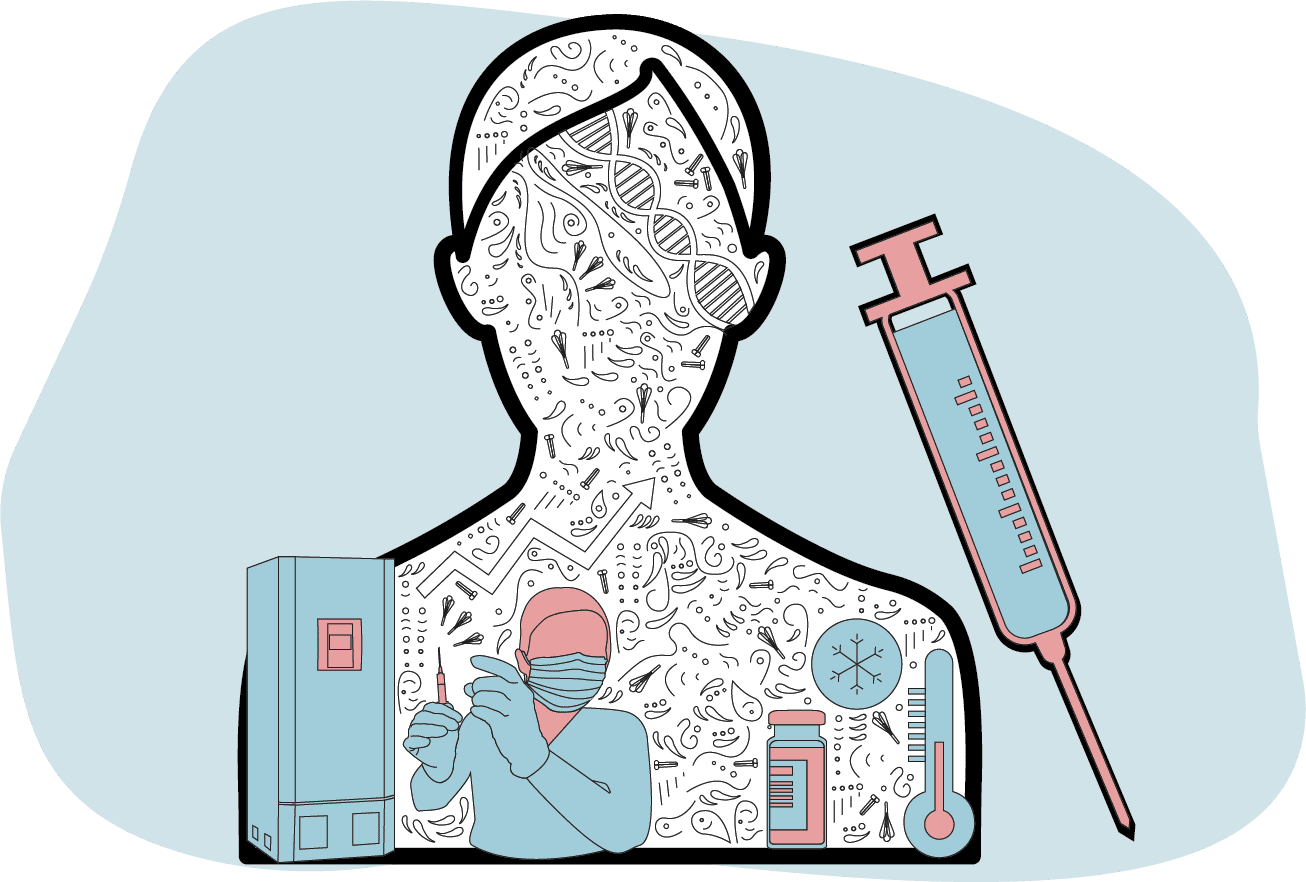
Trained staff at vaccine centres receive these smaller batches and ensure they are stored correctly ready for patients.
Frozen vaccines require defrosting and some also need diluting before being ready for use.
Once in a syringe, the solution is injected into the upper arm of a patient, where its real work begins.
Inside our bodies the vaccine begins to train our immune system to fight coronavirus whenever we encounter it, with the hope of preventing us from getting sick from the devastating disease, Covid-19.
Time, and the close monitoring of those who have received the jab, will then tell us how long that protection is likely to last.
 Will a vaccine give us our old lives back?
Will a vaccine give us our old lives back?
 How do pandemics end?
How do pandemics end?
 Tracking the global coronavirus outbreak
Tracking the global coronavirus outbreak