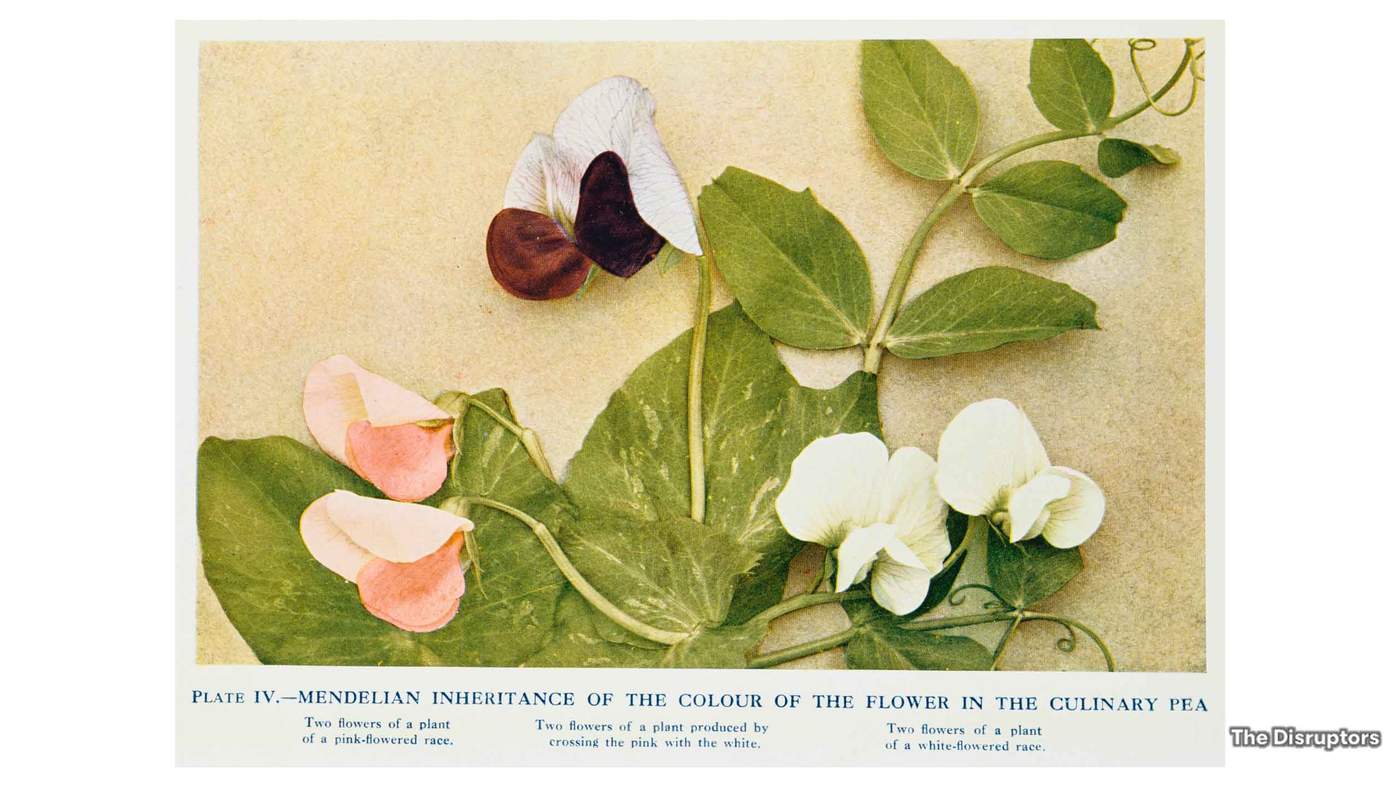
Our quest to understand how our genes work started in earnest in the mid-19th Century when a biologist and monk called Gregor Mendel came to a startling conclusion about the traits of plants. He crossed purple flowered pea plants with white ones, and found that all the resulting offspring were purple. However, he noticed that the third generation produced both colours.

Maurice Wilkins and Rosalind Franklin
This revealed that a characteristic such as colour can be inherited, with one trait more dominant than another. In a way, Mendel had figured out what genes did, but not what they were, or even looked like.
This came much later. It was only in the following century that the very structure of DNA was discovered. Building on the work of Rosalind Franklin and Maurice Wilkins, in 1953 James Watson and Francis Crick discovered that our DNA is formed in a double helix.
Francis Crick
This was a breakthrough. Knowing this structure helped unlock more secrets. When DNA is replicated, this helix splits in two - it "unzips".
This means mutations can be introduced as our cells divide. Even a small genetic error can cause a devastating disease.
In other words, the unique book of letters that makes up each of us can be printed or rewritten with mistakes. But we now have the tools - including the ability to analyse large data sets - to both read our book quicker, cheaper and even tinker with it.
"There are very few examples where new technology has swept across labs around the world, where it's implemented to do things that were extremely difficult to do," says Prof Ali. The use of CRISPR will not, however, be "instant" he warns. It will take a number of years for the technology to be used clinically.
Intellia Therapeutics is one of several companies developing the technology for use in humans. The company's CEO, Nessan Bermingham, believes that CRISPR has the potential to completely revolutionise healthcare.
The eventual hope is that it could target both diseases caused by only one defective gene as well as diseases caused by more than one genetic mutation. "This technology has the potential to allow us to target multiple regions of DNA at the same time," says Bermingham.
Intellia has shown, he explains, that a single injection into an animal can slow down a toxic protein from being produced by 97%.

This is unprecedented in our industry."
Before it can be used in humans, any drugs will have to be extensively trialled and regulated by the relevant authorities. Until then its use will mainly be as a research tool in the lab. "Without doubt, the power [of CRISPR] is how easy it is to edit genomes," says Prof Ali.
Many scientific questions still have to be answered before Intellia can seek approval for clinical trials on humans. For this reason Bermingham is hesitant to propose a specific timeline.
The money though is already flowing. Although Intellia is currently caught up in a battle for the CRISPR patent, Bermingham says this has not deterred investors. "From an investors' standpoint, from a scientific standpoint, people are looking at these discoveries and saying 'now we have the tool, we're ready to go.'"
Potential revolution for healthcare at Intellia
Genome editing also comes with its own scientific controversies. It immediately brings up the question of designer babies. Though it's important to note that altering an individual's DNA will only change the specific genes being edited. The change won't be passed onto their offspring – a process called somatic editing.
It is editing single-celled human embryos that would have an impact on all future generations if they resulted in pregnancy. Trials on human embryos are already taking place, but only for research purposes.
As for Intellia, they are focusing on somatic gene editing. "Any discussion about germ line editing – where those cells or those edits get passed onto your children and their children's children – is premature," says Bermingham.
We will soon see how successful further human trials are, only then will we understand whether CRISPR will be as big a game changer for human disease as it has been predicted to be.
While CRISPR can be used for a range of genetic diseases, including cancer, there are many other companies targeting specific types of cancer. There are over 200 forms of it, making it a very difficult disease to treat.
One emerging technology to treat cancer uses a patient's own immune system to fight it. Our immune system is very efficient at fighting infections. Some of these infection-fighting "machines" in our blood are white blood cells called t-cells, which specifically look for signs of infection. If they spot a virus they multiply and attack.
The problem is that t-cells do not recognise cancerous mutations as invasive enemies, as they are mutated versions of the patient's own cells. "Medical science has long wanted to redirect them from killing virally infected cells to killing cancers," explains Dr Martin Pule of University College London. He and colleagues have now been able to do just that, by genetically altering t-cells to recognise and attack cancerous ones.
One such therapy, called CAR-T, has already been licensed for use in the US, at a cost of $475,000 per patient. The personalized treatment is extremely effective. It treats children and young people with acute lymphoblastic leukaemia and has an 83% remission rate from a single dose, according to Novartis, the company that manufactured the drug. "There has been nothing like it in a generation," says Dr Pule.
These are actually living cells and they go into the patients' cells and graft, they divide, and do all the things they normally do for an infection, killing infected cells."
Dr Pule sees this type of treatment as the future of oncology, with nine clinical trials currently under way at University College London. Several businesses are also now working on treatments harnessing the power of t-cells, these include Autolus and Immunocore, both in the UK, and Novartis in the US.
Immunocore, a company based just outside Oxford, uses a technology called TCR therapy – where a small molecule attracts the t-cells and the cancerous ones. Once both cells are connected, it allows the t-cells to release toxins to kill the cancer.
This molecule has been developed to target a rare type of eye cancer which can quickly spread to the liver. When it does so, the patients do not have long to live. This drug therefore targets these liver tumours. Immunocore has treated 180 patients with promising results.

Eva-Lotta Allan, Immunocore
We have an opportunity to really extend patients' lives."
Eva-Lotta Allan, Immunocore's chief business officer hopes the company will have a drug on the market in the next couple of years. "We have increased the survival rate after one year of treatment by almost four times, compared to other [treatments] out there today," she says. If effective, the technology could also be used to treat infectious diseases such as HIV, TB and autoimmune diseases.
Immunocore's investors, which include the Bill and Melinda Gates foundation and several pharmaceutical companies, have made it possible for them to spend many years working on a drug for such a rare cancer, Allan says. Only about 4,000 patients are diagnosed each year, which could inhibit investment from some quarters. "Major pharmaceutical companies from a commercial perspective may not think it is fruitful to do so."
These are but a handful of the biotech companies which have jumped on the rapidly advancing knowledge of genetics. Investors are keen to cash in on innovative treatments, but profits are not guaranteed and many start-ups will fail in the early rounds. Many companies doing early-stage research, like Immunocore, will not see any returns for many years to come. So why do people invest in medical sciences in the first place?
To start with, if a company's drug or product is successful, the returns can be extremely lucrative, says Hitesh Thakrar, partner at life sciences venture fund, Syncona. Take gene editing. Estimates suggest that CRISPR treatment could cost up to $1m per patient. The UK government also offers tax breaks to invest in qualifying businesses under the Enterprise Investment Scheme. "I invest in start-ups as more innovation is happening from bottom up," says Thakrar.
Monetary goals may be the only factor for some, but Thakrar is also excited by following cutting-edge advances in medicine. "For the first time in the history of medicine we are on the verge of curing people – that's quite an exciting time to be exposed to companies that are curing disease rather than symptoms," he says.
When businesses profit from science it can have other impacts. That so much money is at stake can take funding away from blue-sky research – the pursuit of knowledge where unexpected and ground-breaking discoveries can be made. Some see this shift in research agendas as concerning.
"It's a slightly different pursuit," says Dr Timothy Weil, a molecular biologist at the University of Cambridge. "Many start-ups and venture capitalists are looking to solve a problem, searching for the answer to a known unknown. One of the most exciting aspects of basic, blue-sky research is the prospect of the unknown unknowns," he asserts.
History tells us that just because money is thrown at a problem, it doesn't guarantee you're going to figure it out."
Another issue is that when patents are involved, scientific discoveries are not always shared with other scientists, despite the fact that it is often university researchers who make vital, early discoveries.
Funding for academic research, however, can be time consuming and competitive. Dr Pule splits his time between academia and business. He therefore says that "industrial investment allows the rapid application of large sums of money towards very focused goals." For him, CAR-T is an example where technological and clinical development has advanced really quickly. "It is a good example of the power of capitalism."
What is clear is that investment in emerging technology is growing at a rapid pace. This is why Prof Ali believes that there has never been a more exciting time to be working in biotech. "The influx of investment and establishment of many new companies just demonstrated how much confidence there is that the technology is going to deliver."
It also shows that advances in medicine can and do come from many different areas. "These are generational challenges and the more ways of approaching the problem, the better chance we have to find solutions," says Dr Weil.
It's a long way since Mendel and his pea plants. Rapidly advancing knowledge of the human genome means that these companies - and others like them - will help humans enter into a world where personalised medicine, tailored to individual genomes, will become the norm.