London's clue to stubborn ozone levels
- Published

Dense data: London has a good network of air quality monitoring stations
Scientists think they have identified one key reason why ground-level ozone remains stubbornly high in Europe.
They say it is the unfortunate but unintended consequence of what have otherwise been very successful efforts to improve air quality.
It turns out the filters put on vehicle exhausts to remove fine particulate material have also unbalanced the chemistry behind ozone formation.
Chemical reactions that would normally remove ozone have been subdued.
The insight comes from a study looking at London's air quality records.
"Peak ozone levels have come down since the 1990s, but we haven't had the gains we expected on ozone," said Dr Erika von Schneidemesser from the Institute for Advanced Sustainability Studies, Potsdam, Germany.
"The data we've got from monitoring sites in London, and also the modelling work we have done, has helped us understand why ozone has behaved the way it has - at least in London," she told BBC News.
Dr von Schneidemesser was speaking here at the American Geophysical Union (AGU) Fall Meeting, the world's largest annual gathering of Earth scientists.
Disturbed cycle
Ozone , external in the lower atmosphere (troposphere) is regarded as a serious pollutant that can cause respiratory problems, and even damage masonry and agricultural crops.
The principal originating source, external is the emissions from road vehicles. These include the exhaust gases such as nitrogen oxides (NOx), non-methane volatile organic compounds (NMVOCs), and carbon monoxide (CO).
Ozone is the product of these gases' participation in a complex series of chemical reactions where sunlight and heat act as catalysts. Summer months are generally worse for O3.
Dr Erika von Schneidemesser talks to Jonathan Amos
Dr von Schneidemesser and colleagues used the data from London's dense network of air quality monitoring sites to try to assess the performance of the ozone-producing reactions over the past 15 years.
They found that although the ozone precursors have been falling, the ratio of two NOx gases in the atmosphere has changed.
In constant conditions, there is a neat cycle in which nitrogen dioxide (NO2) helps to form ozone and nitric oxide helps to break it apart. This cycle appears to have been perturbed by control measures that were actually intended to remove the fine particles and black carbon (soot) in vehicle exhausts.
The measures achieved the desired outcome but also altered the relative emissions of the different NOx gases.
"There's this balance between the NO and nitrogen dioxide NO2, and the diesel filters that we've been retrofitting on to things like buses mean that we now have a larger amount of primary NO2 and so you get a reduction in NO that is much greater than the reduction in NO2. This means basically you are taking away some of the ozone suppression," said Dr von Schneidemesser, who is also affiliated to the University of Leicester, UK.
Whereas NO in the atmosphere has been reducing by 5-20% per year, NO2 has been falling by just 1-5% per year.
"As these levels continue to go down, we should then eventually see a reduction in ozone. It's just that the initial steps have had the opposite effect."
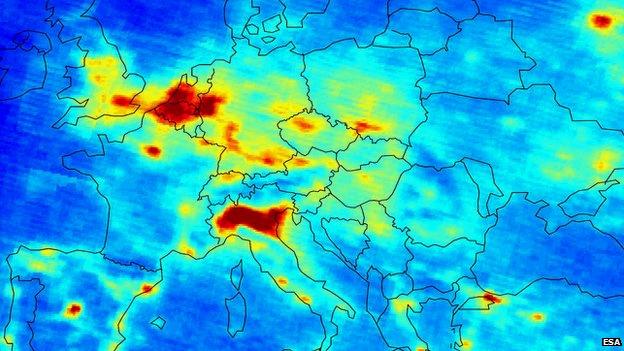
Nitrogen oxides are produced from many sources including power stations, motor vehicles, and industrial and domestic heating systems
Further work is required, but the researchers' suspicion is that London's experience is not unique.
The big traffic-choked cities of Europe will all suffer from similar emissions inventories. The one rider here is that southern European cities will have more sunlight and heat to drive ozone producing reactions.
But the London observations are unlikely to be the whole story. Scientists say it's also that European ozone levels are being influenced by what is happening in other regions of the world.
"There is an import of ozone and precursors from outside, and this influences what we call background ozone; and that's going up as global pollutants, particularly in Asia, go up. And that's affecting European ozone levels," explained co-worker Prof Paul Monks at the University of Leicester.
"So, for something like ozone, we've probably got to move to a more global treaty-like situation. We've got to look at control measures in other countries as well as our own.
"Peak ozone has gone down since the 1990s, but it has bottomed out now; and it's remaining fairly flat despite emissions reductions."
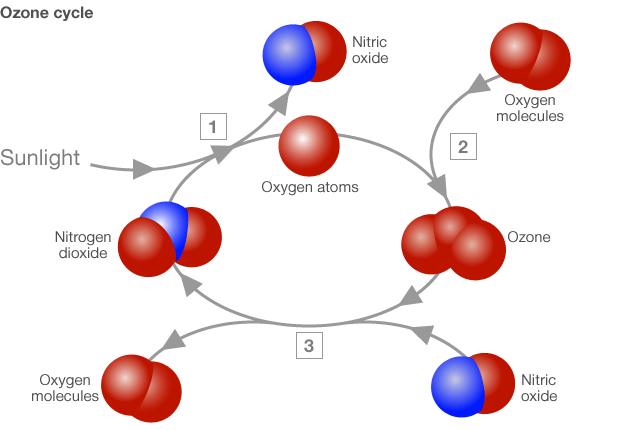
Ozone's NOx cycle
(1) The Sun's ultraviolet light breaks oxygen atoms off nitrogen dioxide molecules
(2) Oxygen atoms then react with oxygen molecules in the air to produce the ozone
(3) But ozone is destroyed by nitric oxide, reforming molecular oxygen and nitrogen dioxide
Jonathan.Amos-INTERNET@bbc.co.uk and follow me on Twitter: @BBCAmos, external
- Published5 December 2012
- Published8 August 2012
- Published8 December 2011