150 years of the periodic table: Test your knowledge
- Published
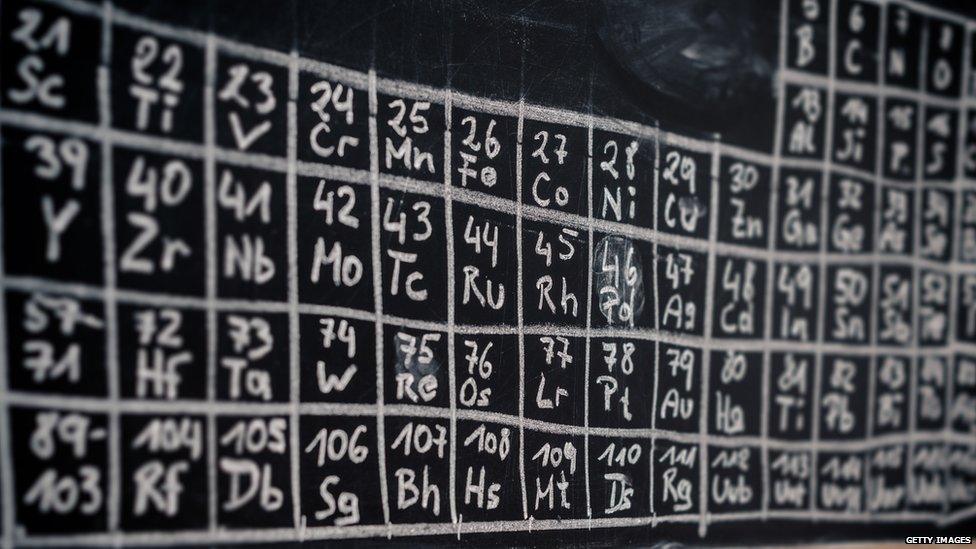
You'll find it on the wall of nearly every school chemistry laboratory in the land.
And generations of children have sung the words, "hydrogen and helium, lithium, beryllium..." in an attempt to memorise some of the 118 elements.
This year, the periodic table of chemical elements celebrates its 150th birthday.
As the candles on the cake are lit, it's time to test your knowledge, with our quiz.
The United Nations has designated 2019 as the International Year of the Periodic Table, external to celebrate "one of the most significant achievements in science".
In March, it will be 150 years since the Russian scientist, Dmitri Mendeleev, took all of the known elements and arranged them into a table.
Most of his ideas have stood the test of time, despite being conceived long before we knew much about the stuff that makes up matter.
On Tuesday, the year will be officially launched, external in Paris. So, what's so special about this iconic symbol of science?
Alien concept?
Dr Peter Wothers of the University of Cambridge is an expert on the subject.
Chemist Dr Peter Wothers runs courses on the periodic table
He thinks if aliens came down to Earth, this flag of science would not have escaped their attention.
"Would an alien have a periodic table?" he says.
"I think they probably would, because it is something that is absolutely fundamental - this is not just some creation of humans, there is something innate and fundamental to this - there's chemical law, physical law behind this."
The laws of chemistry
Mendeleev (1834-1907) created his early periodic table in 1869. He took the 63 known elements and arranged them into a table, mainly by their atomic mass.
Although he wasn't the first to do this, his interpretation involved a leap of ingenuity, in that he put those with similar properties below each other into groups and left gaps for new elements to be slotted in.
"People had been doing that for some time - but finally there was some natural basis - or some law - that meant they needed to be arranged in some way," says Dr Wothers.
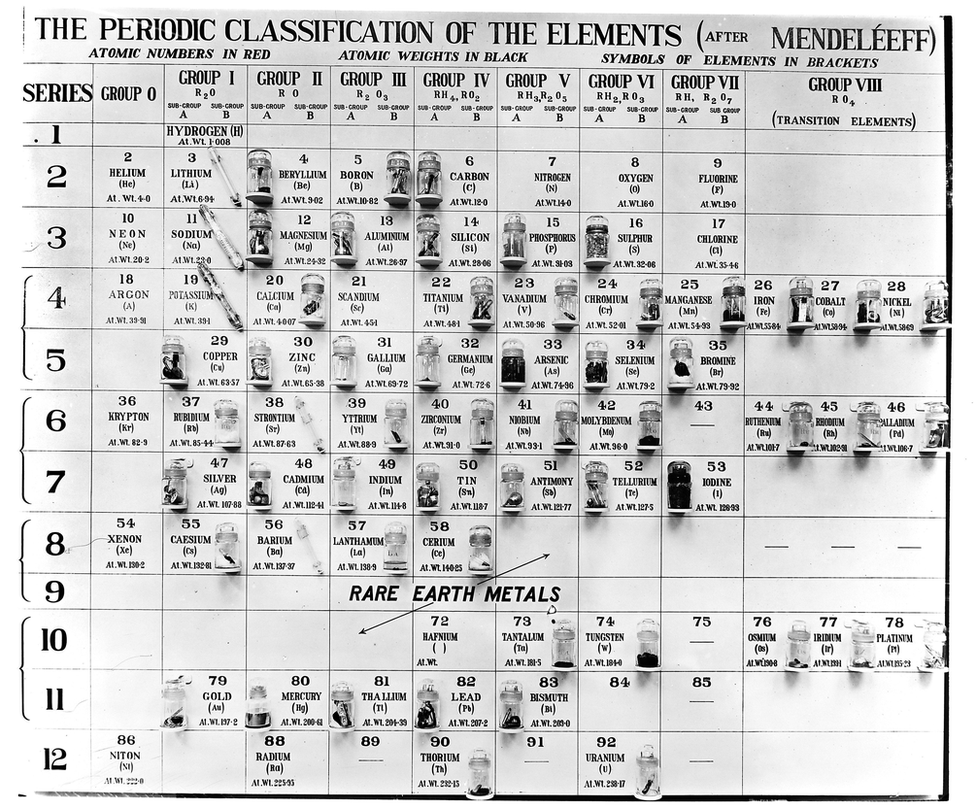
A version of the periodic table based on Mendeleev's original table
One hundred and fifty years on, there have been fundamental shifts in our understanding of matter.
"Obviously Mendeleev at the time knew nothing about the sub atomic structure of the atom, so he was going on only the atomic weights, which weren't necessarily determined to the right accuracy at the time," says Dr Wothers.
After the discovery of protons, scientists realised that the atomic number of an element is the same as the number of protons in its nucleus. Thus, in the modern periodic table, the elements are arranged according to their atomic number - not their relative atomic mass.
"We now know the 'how it works, why it works', and this is to do with quantum mechanics and the arrangements of electrons in atoms and so on," he says.
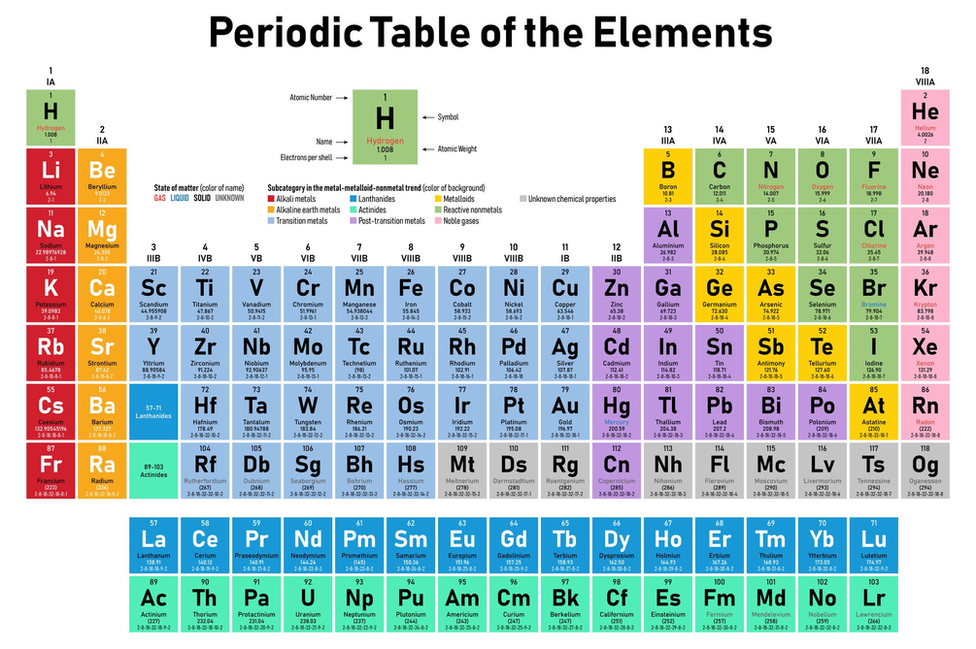
The Periodic Table we know today
There are now more than 100 elements, laid out in order of increasing atomic number. There are repeating patterns in the properties of the elements, which give the periodic table its name.
Elements with similar properties are arranged to form columns (groups).
The periodic table is now a thing of both beauty and practical use, says Dr Wothers.
"You can understand certain things just by considering the place of an element in this table, or in this arrangement, that's why it's so useful to chemists."
The joy of symmetry
This year, which has been designated the International Year of The Periodic Table, may also represent its heyday.
Currently, the seventh period of the periodic table has been completed, with the recent addition of four elements in December 2015.
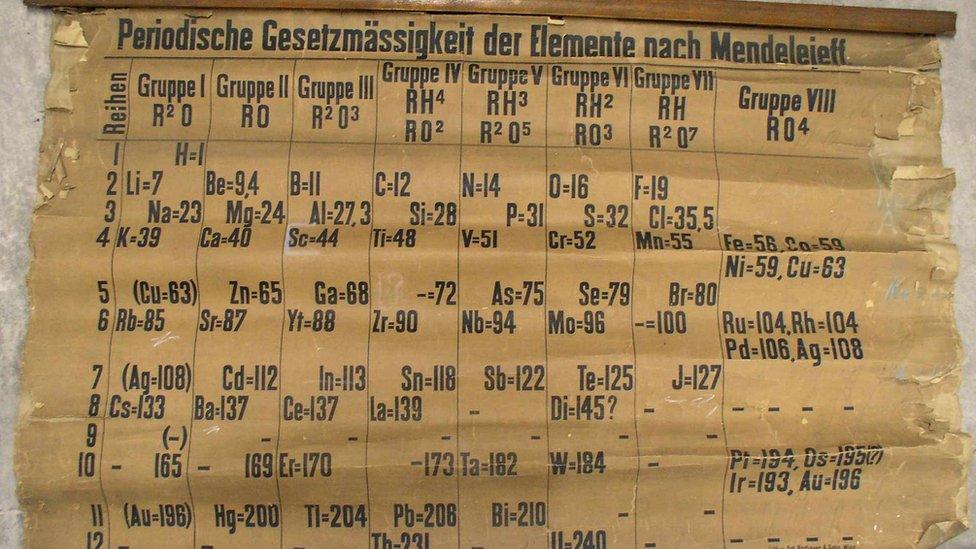
Early periodic table chart from around 1879 recently discovered at the University of St Andrews
This has made it "quite whole and beautiful", he says.
"At the moment - this very year - I think we are very privileged, because the periodic table is in its most perfect form, " says Dr Wothers.
"And probably the most perfect form it will ever be in."
People are currently working on synthesising heavier elements, and, if they manage the task, the periodic table will change yet again.
"As soon as just one more is discovered, then we'll have to start a row - the eighth period," he says.
"And then it will lose some of its beauty, because the eighth period will never be completed I think it's fair to say."
Follow Helen on Twitter, external.
- Published17 January 2019
