'My husband squeezed my hand to say he wanted to live, then I found a way to save him'
- Published

Tom and Steffanie, with illustrations of Acinetobacter and a bacteria-eating phage

When Tom Patterson started vomiting during an Egyptian holiday he thought he had food poisoning. He was wrong. In fact he was infected with an antibiotic-resistant superbug and only his wife's determination and a revolutionary new treatment would save him.
"Has anyone told Steff her husband is going to die?"
It was a question Steffanie Strathdee was not supposed to hear. She had been talking on the telephone with colleagues, who continued talking among themselves after they thought she'd hung up.
Steffanie, an infectious disease epidemiologist, knew her husband, Tom Patterson, was desperately ill - he was at that moment lying in a medically induced coma - but it still came as a shock to hear that he was now expected to die.
"I thought, 'Oh my God. No, nobody has.' I cradled the phone in my arms like a baby, and that moment was so profound. I realised if he's really dying I need to do something. If modern medicine doesn't have anything left I need to try my very best."
Doctors were quickly running out of ways to keep Tom alive, as a deadly superbug rampaged through his bloodstream - resistant to all antibiotics they had to offer.
Steffanie had read in some medical literature that sometimes people in comas can hear, so she decided to ask Tom if he wanted to live.
"I thought I can't just take matters into my own hands and keep him alive if he doesn't want to live any more. I need to ask him," Steffanie says. "So I held his hand with my blue-gloved hand and I said, 'Honey, if you wanna live, you need to give it all you've got, the doctors don't have anything left. All these antibiotics are useless now. So if you want to live, please squeeze my hand and I will leave no stone unturned.'"
After a while, Tom squeezed her hand.
"I pumped my fist into the air and said, 'Oh this is wonderful!'" Steffanie says. "And then I realised, 'Oh my God, what am I going to do now? I'm not a medical doctor, I don't know what to do.'

The couple, both scientists at the University of California, San Diego, had met through their work in Aids research. Avid travellers, they visited about 50 countries together, often tacking on a few days of holiday at the end of academic conferences to explore new places.
In November 2015, they were about to travel to Egypt, when a terrorist bomb brought down a Russian jet flying out of Sharm el-Sheikh. They discussed whether to cancel the trip, but decided to go anyway.
"Tom said, 'Oh it's the perfect time to go! There won't be any crowds!' I said, 'Are you crazy?' I wrote out the rest of our will by hand and I handed it to my parents, who were house-sitting. We thought that if there was going to be a problem, it would be a terrorist attack or something."
The trip was fantastic, as expected. Their last stop was the Valley of the Kings, and to get there, they took an overnight boat ride down the Nile. Almost the only passengers on a riverboat designed for 150, they had a wonderful meal under the stars on the deck of the ship, the Nile shimmering all around them.

But once back in their cabin, Tom started vomiting. At first the couple assumed it was food poisoning. On their travels they always carried Cipro, an antibiotic, but this time it didn't work. Tom kept getting worse, and started experiencing pain in his back. It didn't feel like the food poisoning he'd had before.
Steffanie got Tom to some doctors on dry land, who carried out a CT scan and discovered that it wasn't food poisoning at all. He had an abcess in his gut, known as pseudocyst, which had grown nearly to the size of a football.
Thanks to their medical insurance, taken out for $35 before the trip, Tom was med-evaced to Frankfurt, where doctors found that the initial cause of the problem was a stone expelled from his gallbladder that had got stuck in his biliary duct. Inside the cyst they found a murky, brown fluid that indicated this wasn't a new infection. As they worked to figure out what was happening, Tom started to fall into a coma.
"I was hallucinating, thinking I was in Egypt, seeing hieroglyphs on the walls, really losing it," Tom says. "Because of the infection in my gut - and by this point I hadn't had much sleep in a few days - I was getting pretty crazy. The doctors came back and said, 'This is the worst infection on the planet. This is an infection that's closed down hospitals in Germany. It's called Acinetobacter baumannii.'"

Tom was put into isolation, and his children flew over because of concern that he wouldn't make it. When doctors visited him, they wore special gowns.
This startled Steffanie, who was familiar with Acinetobacter from her undergraduate studies in microbiology.
"I was really shocked because this is an organism that I used to plate on my petri dishes back in the 1980s, and it was considered to be a very wimpy pathogen back then. We just needed gloves, a lab coat and no special equipment," she says.
"Over the last couple of decades, it's become what is essentially a bacterial kleptomaniac. It's learned how to steal antibiotic resistance genes from other bacteria and it has taken on these superpower capabilities that have made it a very deadly pathogen."
In 2017 it was listed by the World Health Organization as one of three superbugs for which new antibiotics were most urgently needed. Fortunately, there were still some antibiotics that worked on Tom, though, and the Frankfurt medical team was able to stabilise him.

Find out more

Tom and Steffanie were friends, through their work, with a number of medical experts who were able to advise on Tom's situation, and it was decided to move him back to San Diego. Doctors there had experience of Acinetobacter baumannii, due to the high military presence in the area - the bug has been nicknamed Iraqibacter, because of the large number of infections picked up by US forces in the Middle East.
When Tom arrived, he was tested again for sensitivity to antibiotics. It was bad news. None of them were now having any effect.
The doctors had a tough decision to make: they could either operate to remove the abcess, or try to siphon the infected fluid out of his body. But operating, it was decided, was too risky - if the organism got into his bloodstream he would go into septic shock.
Steffanie describes septic shock as the overreaction of the body's immune system to the invader. The body goes into "red alert", blood pressure drops, heart rate increases, breathing speeds up.
"It happens very quickly and has a 50% mortality rate," she says.
So doctors opted for siphoning off the fluid instead, poking five drains into Tom's abdomen.
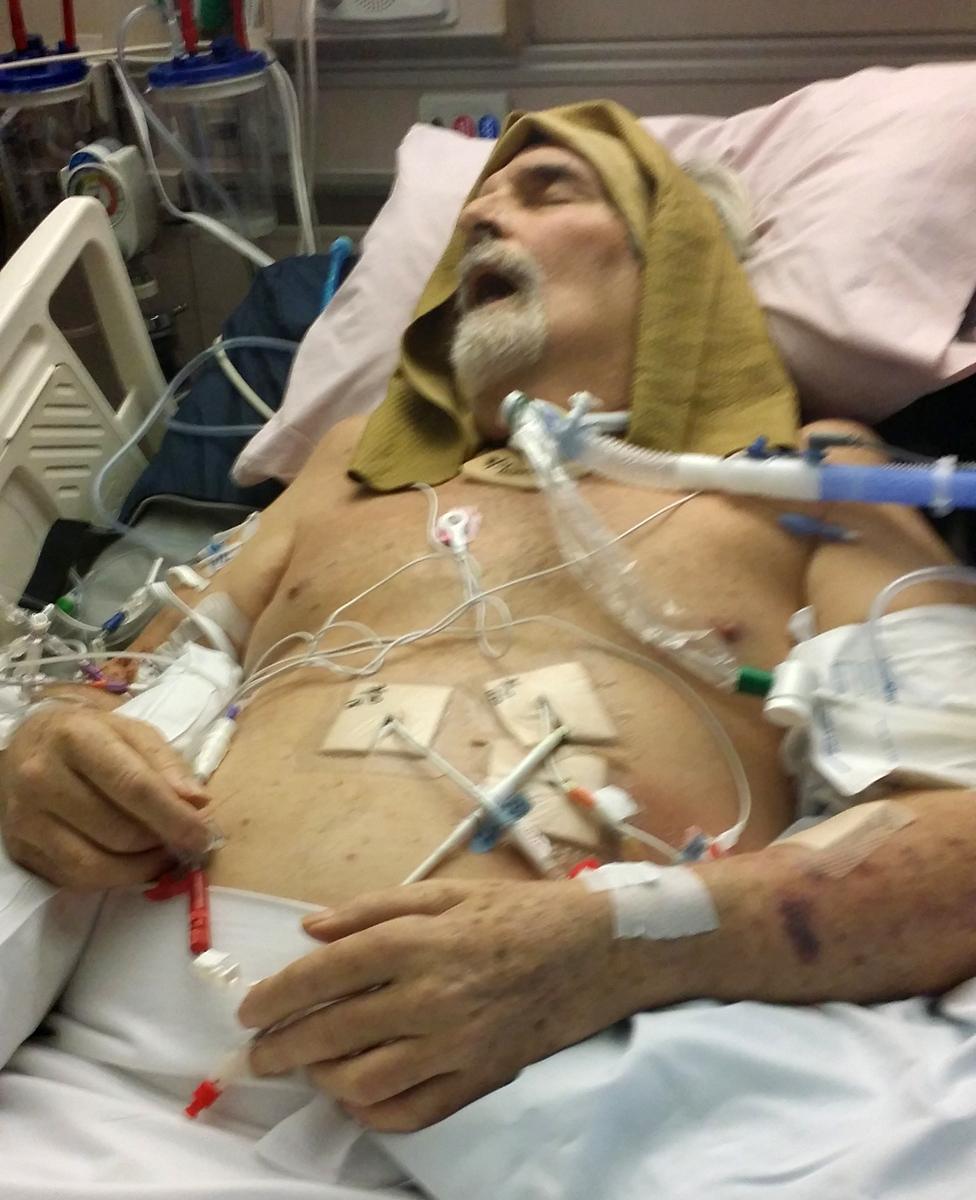
Plans were made to transfer him to a long-term acute care facility. However, the day before this was due to happen, one of the drains slipped as he tried to sit up in bed, dumping all the infection into his bloodstream. He immediately went into septic shock, was rushed back into intensive care and put on a ventilator to breathe.
"From that moment on, the bacteria was colonised everywhere in his body - in his blood, not just his abdomen. He was slipping away day by day," Steffanie says.
A large man, 6ft 5in tall and weighing 300lb (21 stone), he had already lost a huge amount of weight.
"I could put my fist into the hollow in his cheekbone and two knuckles behind the orbits of his eyes and it was just horrible," Steffanie says.
At this point, Tom didn't really know what was happening. "I was hallucinating these elaborate stories that were almost biblical in proportion. Things like I spent 100 years wandering through the desert trying to answer three questions posed by holy men. That went on for days," he says.
He would come out of his coma for a short time, and was then able to communicate with the people around him, but he couldn't get out of bed.

It was around this time that Steffanie overheard her colleagues asking if she'd been told Tom was going to die - and that she asked him to squeeze her hand if he wanted to live.
What Steffanie didn't know was that at this moment he was hallucinating that he was a snake. How could he squeeze her hand when snakes don't have hands? He eventually worked out that he could wrap his whole body around her hand - only then did he give the signal.
Realising that desperate measures were called for, Steffanie turned to PubMed - the search engine of the National Library of Medicine.
"I put in 'multidrug resistance', 'Acinetobacter baumannii', and 'alternative treatments', and up popped a paper with something called bacteriophage therapy in the title and I thought, 'Bacteriophage... I remember what they are.'"
Phages are viruses that have naturally evolved to attack bacteria and, again, Steffanie had studied them for a short time as an undergraduate.
They are tiny, 100 times smaller than bacteria, and they are everywhere, she says, in water, in soil and on our skin. It's estimated that 30 billion of them pass in and out of our bodies every day.
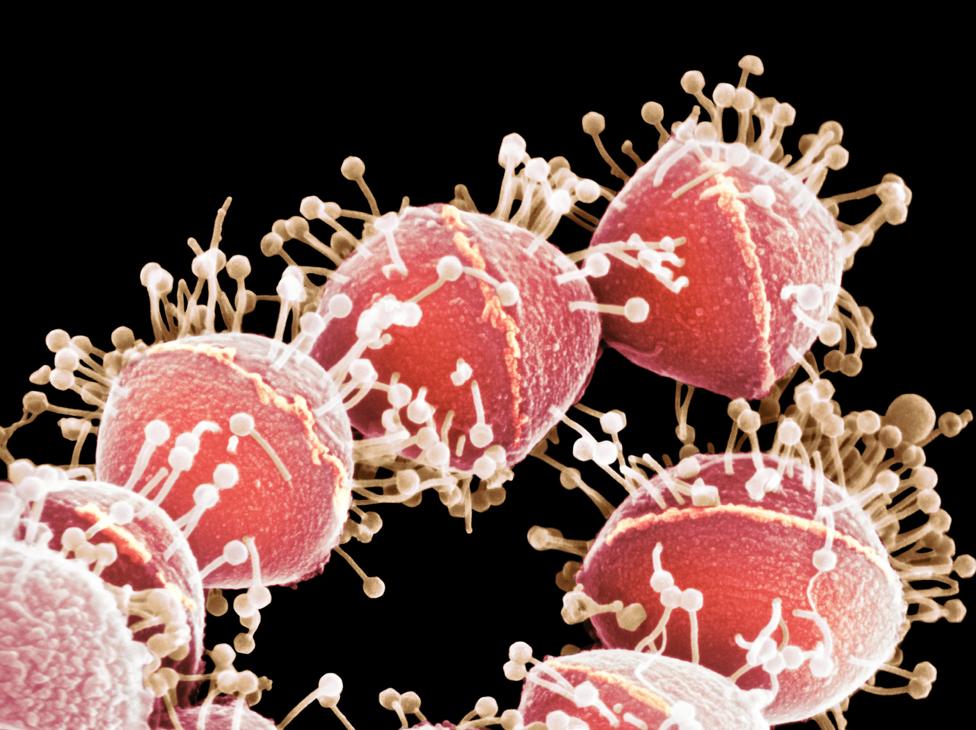
Phages seen attacking Streptococcus bacteria - in a coloured image from a scanning electron micrograph
A century ago, phages were attracting a lot of attention as a possible cure for bacterial infections, but outside the former Soviet Union and parts of Eastern Europe this research fell by the wayside after the discovery of the wonder drug, penicillin, in 1928.
After penicillin came other kinds of antibiotics, says Steffanie, which made doctors in the West think they could always find a fix for any new bacterial infection.
"Boy were we wrong!" she says. "It was only when these superbugs, these bacteria that have been resistant to multiple antibiotics, started to become a real global health threat that we started to take a second look [at phages]."
Steffanie's next step was to approach the Food and Drug Administration, with the help of one of Tom's physicians, Dr Chip Schooley of the University of California San Diego Department of Medicine, which approved an experimental treatment on compassionate grounds.
But there was a catch. In order for the treatment to work, Steffanie had to find phages that matched the particular form of the Acinetobacter bacterium Tom was infected with - and with trillions of phages on the planet this was no easy task.

Steffanie turned back to the internet and contacted researchers in North America she hoped would be able to help - though none were using phages as a medical treatment.
Dr Ry Young at Texas A&M university replied quickly, offering to turn his lab into a command centre, and asking phage experts from all over the world to send him their phages to be tested against Tom's bacteria.
"We essentially had phage researchers from all over the world who were offering help - from Switzerland, Belgium, Poland, the Republic of Georgia, India. The Belgians even offered for their phage to be sent in a diplomatic pouch. These were total strangers who had stepped up to the plate, a true global village to rescue one man," Steffanie says.
Within three weeks, thanks partly to a PhD student who slept in the lab to do vital work around the clock, a cocktail of four phages was ready.
By this point, Tom was on full life support. His lungs and kidneys were failing, he was on a ventilator, and required three different medications to keep his heart pumping. He may have been only hours away from dying.
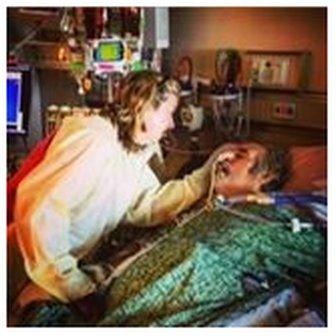
Steffanie wore protective clothing when visiting Tom, to reduce the risk of the superbug infecting other patients
The atmosphere in the hospital was becoming emotional, even spiritual.
"People were lighting candles, saying prayers, sending song recommendations. We had music playing in the background and Tom even remembers to this day The Beatles playing when he was lapsing in and out of coma," says Steffanie.
The responsibility for the experimental treatment they were about to undertake weighed heavily on her.
"It was terrifying because I thought, 'Well he's going to die anyway, if we don't do this… but if this kills him I'll have to bear this on my conscience for the rest of my life.'"
The first phage cocktail was injected into tubes in Tom's abdomen, closest to the infection. When he was more stable, a second and more potent phage cocktail, developed in a US Navy medical centre, was injected into his bloodstream.
This was a real innovation, as even in places where phage therapy is still used, it's not usually administered intravenously. The phage preparations could kill the patient if they aren't clean enough, and often they come from dirty places rich in bacteria, such as sewers - "from some of the gnarliest places you can imagine," as Steffanie puts it.
But three days after the phages were administered, Tom woke up from his coma.
"My daughter was standing over me and I reached out for her hand and kissed it," he says. "I couldn't speak at the time and then I was tired, I fell back asleep."

Soon after the start of the phage therapy Tom had another case of septic shock, his sixth out of seven in total during the nine months he spent in hospital.
A number of phages were used as his treatment continued, and the bacteria developed resistance to some of them. It's not completely clear which phages worked and which didn't.
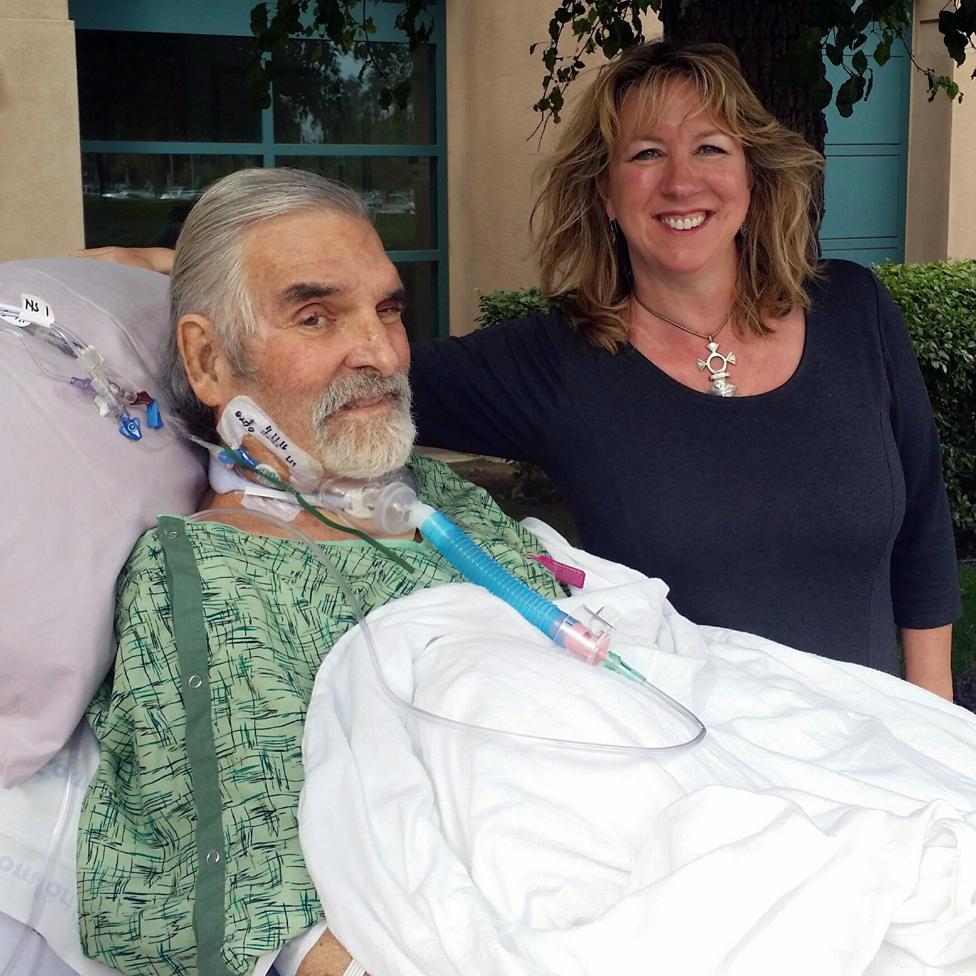
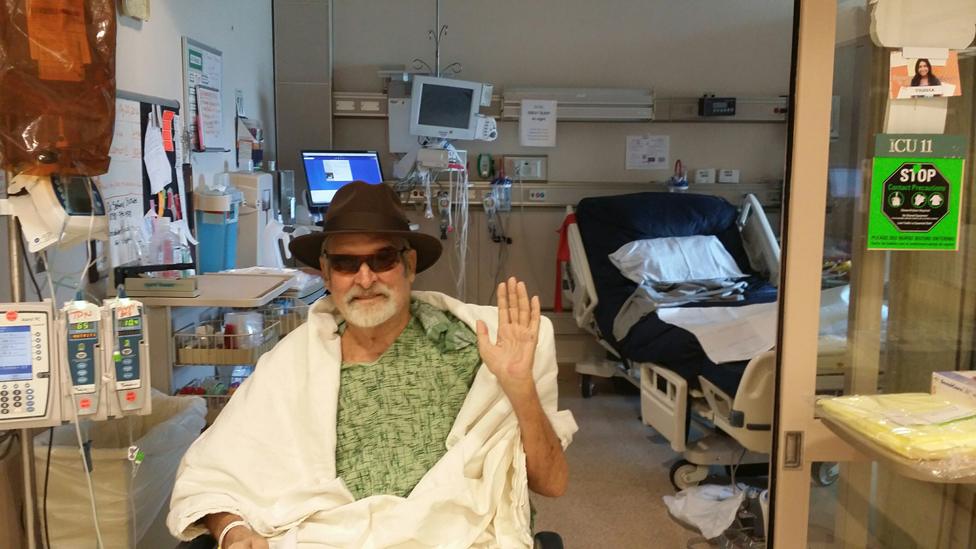
Tom's first trip outside into fresh air
He's now three-quarters of the way through a predicted four-year recovery period.
He had to re-learn to swallow, to talk, to stand, to walk. He left hospital in a wheelchair because his muscles had wasted away.
One of the lessons Tom draws from his months in hospital is about the importance of company. His son-in-law put together a schedule of visitors to ensure that someone was with him 24/7, and even when he was in a coma, he could often hear, at a distance, what was going on.
"I could hear people talking, and people read to me and sang to me, and held my hand - and a touch was like an electric shock, so much energy comes to you," he says.
Tom was the first person in North America to receive intravenous phage therapy to treat a systematic superbug infection. Steffanie recognises how lucky they were, and how much depended on her connections, which enabled her to launch the international effort to save her husband.
When, a year into Tom's recovery, his case was presented at the Pasteur Institute in Paris, at a gathering to mark the 100th anniversary of the discovery of the bacteriophage in 1917, Steffanie started receiving calls from people all over the world - people whose family members who were dying from a superbug infection, and who wanted to try phage therapy.
Saying saying goodbye to the hospital bed
"I was overwhelmed," she says. "But I tried to recreate the same kind of response that was made for Tom. We saved some people, not just their lives but their limbs, and one of the most miraculous cases to have occurred as a result of Tom's was Isabelle."
Isabelle Carnell-Holdaway, a British teenager suffering from cystic fibrosis, had developed an antibiotic-resistant infection after a lung transplant. In October 2017, Isabelle's doctors got in touch with Graham Hatfull, a phage expert at the University of Pittsburgh, and his team used their vast phage collection to develop a genetically modified phage cocktail to treat Isabelle. Dr Chip Schooley, who presided over Tom's phage treatment, then worked with the Pittsburgh and London teams to gain approval for the therapeutic use of the cocktail.
Therapy began in June 2018, and Isabelle soon began to recover. Within months she was able to return to a normal routine, even though she had once been given only a 1% chance of survival. The experience gained from saving Tom was invaluable for treating Isabelle's infection.
There are still many hurdles to cross before phage therapy can enter mainstream medicine.
Phages are not like drugs, where one drug might be active against a wide variety of organisms. Phages work best when they are tailored very precisely to the bacterium a particular patient is infected with, which makes designing clinical trials more complex. So far only a few have taken place.
But Steffanie and Tom have become phage evangelists. They have told their story in a book, The Perfect Predator, which is now being made into a documentary and a Hollywood film.

Tom and Steffanie on discharge day
They have also opened the Center for Innovative Phage Therapy and Therapeutics at the University of California, San Diego - the first dedicated phage therapy centre in North America.
Part of their mission is to persuade people of the urgency of finding a solution to antibiotic resistance. Unless something is done, Steffanie says, one person will be dying of a superbug infection every three seconds by 2050.
"As an infectious disease epidemiologist, having my husband dying from a superbug was just a shock," Steffanie says.
"It felt like God's cruel joke. Part of me was the scientist trying to analyse things and get control, the other part of me was the wife-me, trying to hold my husband's hand and cope with a desperate situation.
"But to be honest, I was extremely embarrassed because I was really blind to this global threat - the superbug crisis - that had crept up on me."
All pictures courtesy of Steffanie Strathdee unless otherwise specified
You may also be interested in:

Because of rare illness, Louise Moorhouse is on a special diet of pills or foul-tasting shakes. There's a drug that would allow her to eat like anyone else - she took it for three years during a clinical trial. But the NHS won't pay for it, and the drug company stopped giving it to her once the trial was over.
'I helped test a wonder drug - then I was denied it' (April 2019)