Tiny solar cells fix themselves
- Published
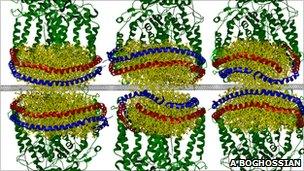
A computer simulation shows how the parts assemble themselves spontaneously
Researchers have demonstrated tiny solar cells just billionths of a metre across that can repair themselves, extending their useful lifetime.
The cells make use of proteins from the machinery of plants, turning sunlight into electric charges that can do work.
The cells simply assemble themselves from a mixture of the proteins, minute tubes of carbon and other materials.
The self-repairing mechanism, reported in Nature Chemistry, could lead to much longer-lasting solar cells.
The design and improvement of solar cells is one of the most vibrant areas of science, in part because sunlight is far and away the planet's most abundant renewable energy source.
More than that, nature has already proven that sunlight can be captured and turned into other forms of energy not only with extraordinary efficiency but also with a self-repair mechanism that counteracts the ravages of sunlight.
"Sunlight, when it hits oxygen, is very damaging," explained Michael Strano, the Massachusetts Institute of Technology chemical engineer who led the research.
"It's the reason why we age, and the reason why when we leave paper or plastic out in the sun, it fades."
The destructive mixture of sunlight and oxygen, Professor Strano told BBC News, means that many of the best solar cells in the laboratory might not survive well when put into use.
"There's a kind of a horse race among scientists around the world to make the highest efficiency cell, but very few people are asking what happens with that cell when you plug it in for a few hours or for a week or for months," he said.
'Jigsaw puzzle'
Now Professor Strano and his colleagues have made novel use of the photosynthetic reaction centre, one of the plant parts nature has developed for the task, in a bid to increase the lifetimes of solar cells.
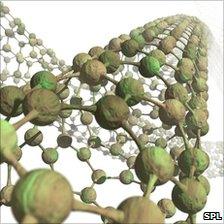
The nanotubes are scaffolds on which the light-sensitive proteins can build
They also employed lipids, the molecules that pair up end-to-end form much of the walls of all living cells, and carbon nanotubes, tiny "straws" of pure carbon that are renowned for their electrical properties.
Lastly they added a surfactant - a molecule that, like soap on grease, breaks certain molecules apart and keeps them separate.
To the team's surprise, this cocktail of disparate parts, when the surfactant was pumped out, assembled itself into a suite of working solar cells, each just a few nanometres - billionths of a metre - across.
The lipids paired up to form discs that attached to the nanotube on one side and to the reaction centres on the other.
Incoming light is gathered by the reaction centre, knocking free an electron that is channelled by the lipids and into the nanotube.
Inside what is known as a photoelectrochemical cell, those electrons can be scooped up and together constitute an electric current.
"It's like a jigsaw puzzle that you throw into the air and it comes down completely assembled," Professor Strano said.
This self-assembly leads naturally to a self-repair scheme.
Surfactant is added, along with a few proteins to replace those damaged by sunlight, and the recipe is complete.
When the surfactant is removed, the bits re-assemble into a pristine set of solar cells.
Professor Strano said that the efficiency of the cells as designed is just a tiny fraction of that provided by the current best solar cells.
While he said great gains are still to be had in efficiency as the experiment is refined, he added that the idea behind the research was as important for future work.
"What our paper is good for is starting to think about device lifetime and borrowing concepts from nature. Can we make cells that have an infinite lifetime?"
What is a nanometre? Professor Tony Ryan explains