Modern Meadow aims to print raw meat using bioprinter
- Published

In the future, your beef may come from a printer, not a cow
When you buy some beef at the butcher's, you know it comes from cattle that once mooed and chewed.
But imagine if this cut of meat, just perfect for your Sunday dinner, had been made from scratch - without slaughtering any animal.
US start-up Modern Meadow believes it can do just that - by making artificial raw meat using a 3D bioprinter.
Peter Thiel, one of Silicon Valley's most prominent venture capitalists, Paypal co-founder and early Facebook investor, has just backed the company with $350,000 (£218,000).
Set up by father-son team Gabor and Andras Forgacs, the start-up wants to take 3D printing to a whole new level.
For three-dimensional printing, solid objects are made from a digital model. It's also known as additive manufacturing: to make the structure tiny droplets are "printed" - layer by layer - via a carefully controlled inkjet nozzle.
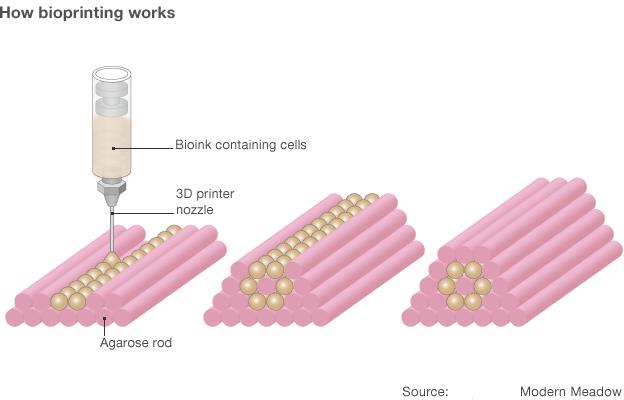
Bioink containing various types of cell is printed into moulds made from agarose gel.
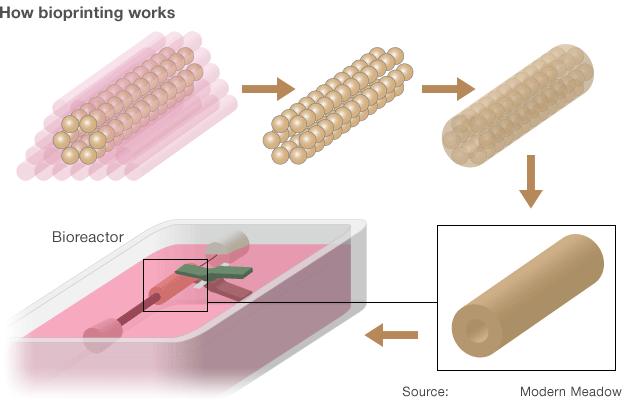
After several days the bioink fuses and the agarose support is removed. The tissue is put into a bioreactor and given low frequency stimulation to mature the muscle fibres.
The principle has been around for more than a decade, and is already used successfully to create jewellery, toys, furniture, cars, and even - most recently - parts of a gun.
Some researchers have also managed to print food like chocolate.
But Prof Gabor Forgacs, of the University of Missouri, says bioprinting something that is part of a living creature is much more challenging than making an earring or a chocolate bar.
"We are printing live material - [the] cells are alive when we are printing them," he says.
"Three-dimensional printing has taken off big time, and printing things such as whipped cream is just another application of it - but it's no big deal.
"Printing biomaterial is an entirely different ball game."
Prof Forgacs says that he and his team have already managed to produce a prototype, but it is not yet suitable for consumption.
Regenerative medicine
To bioengineer meat, the scientists first get stem cells or other specialised cells from an animal via a common procedure known as biopsy.
Stem cells are cells able to replicate themselves many times, and also can turn into other specialised cells. Once the cells multiplied to sufficient numbers, they are put into a bio-cartridge.
So instead of traditional ink or a material like plastic, the 3D printer cartridge contains something called bioink made of hundreds of thousands of live cells.
Once printed in the desired shape, the bioink particles naturally fuse to form living tissue.
Hod Lipson: 'People have been trying to expand the range of materials that can be fabricated using a 3D printer'
This process of bioprinting biomaterials is similar to attempts to print artificial organs for transplants - but the result could well end up in your frying pan.
So far there have been trials using bioprinted tissue and body parts have only been done on animals.
"In some sense, Modern Meadow is taking the technology beyond regenerative medicine," says Prof Forgacs.
Before Modern Meadow, he co-founded Organovo - one of the firms pioneering the use of printed live structures for medical purposes.
In 2010, Organovo successfully bioprinted functional blood vessels made from the cells of an individual person.
Another team of researchers, led by Jeremy Mao at Columbia University, implanted a 3D-printed tooth-shaped scaffold into the jawbone of a rat, and showed that the animal started naturally growing a tooth using stem cells from the body within weeks.
Researchers at Wake Forest University in North Carolina, working with the US Armed Forces Institute for Regenerative Medicine, have bioprinted cells directly onto skin wounds of mice, to accelerate the healing process.
Post-mortem tissue
But many aspects of regenerative medicine are still to be perfected before any human trials start.
"When you want to engineer an organ you have a zillion conditions and requirements to fulfil," says Prof Forgacs.

Scientists in the Netherlands have grown strips of muscle in their lab
"You have to be extremely careful as a tissue or an organ are very complex structures.
"In the case of meat, if you think about a hamburger, its lateral dimensions are much bigger than its thickness so that makes the printing considerably simpler.
"So we're not dealing with incredibly complex 3D shapes, intertwined channels, and so on - we want to build something that has this quasi-2D shape."
The main similarity between engineering organs and meat is that in both cases, the result is made of biological material - except that the latter is post-mortem tissue.
"It eventually will be killed - not killed in the sense of killing an animal but killing the tissue construct," says Prof Forgacs.
Although the actual process of making meat may be simpler, it will be challenging to produce such meat on an industrial scale, and persuade consumers to accept it, he adds.
"We're still struggling with coming out with the right term for our meat.
"You say 'engineered' or 'lab-made' meat, and the folks on the street probably are not going to be very happy to hear that."
Pricy burger
It is not the only attempt under way to create a piece of synthetic meat.
Researchers at Maastricht University in the Netherlands are growing animal cells to produce strips of muscle tissue.
Project leader Mark Post says that the team is creating what could become world's first artificial hamburger, aiming to demonstrate it to the public later this year.
His team does not use bioprinting, but a form of biofabrication where the stem cells multiply in a specially prepared scaffold, effectively engineering live tissue.
The Dutch team has already showcased small pieces of artificially grown muscle about 2cm long, 1cm wide and about 1mm thick.
Producing a whole hamburger using his method would currently cost about £200,000, he says, but the price will plummet as the technology advances.
So in the future, more cows may be able to roam the fields without having to fear the slaughterhouse.
- Published19 February 2012
- Published16 September 2011
- Published8 March 2011
- Published21 February 2011