Brain implants used to fight drug addiction in US
- Published
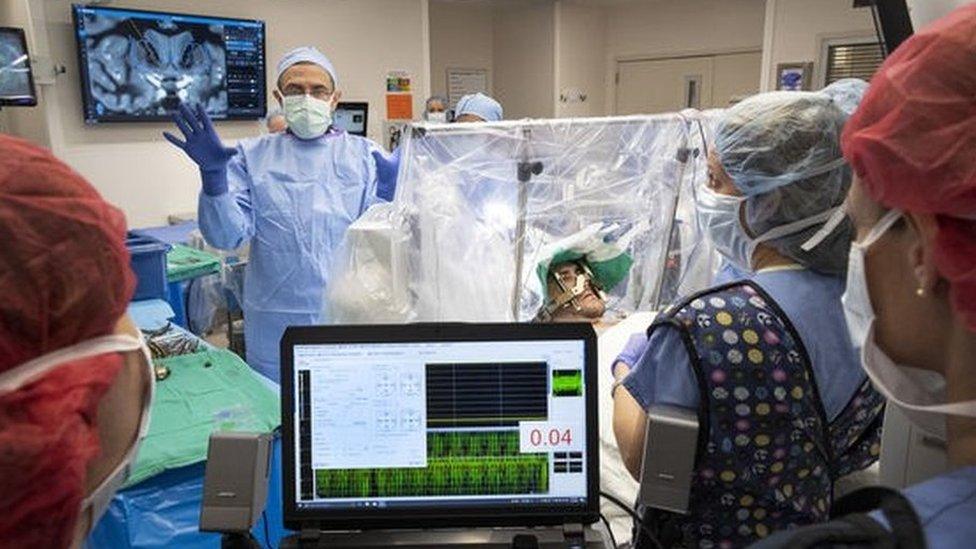
Dr Ali Rezai and his team performed the procedure earlier this month
Patients with severe opioid addiction are being given brain implants to help reduce their cravings, in the first trial of its kind in the US.
Gerod Buckhalter, 33, who has struggled with substance abuse for more than a decade with many relapses and overdoses, has already had the surgery.
Lead doctor Ali Rezai described the device as a "pacemaker for the brain".
But he added it was not a consumer technology and should not be used for "augmenting humans".

Gerod Buckhalter has struggled with addiction for years after being prescribed opioids for a football injury when he was 18
Mr Buckhalter had his operation on 1 November at the West Virginia University Medicine Hospital. Three more volunteers will also have the procedure.
It starts with a series of brain scans. Surgery follows with doctors making a small hole in the skull in order to insert a tiny 1mm electrode in the specific area of the brain that regulate impulses such as addiction and self-control.
A battery is inserted under the collarbone, and brain activity will then be remotely monitored by the team of physicians, psychologists and addiction experts to see if the cravings recede.
So-called deep brain stimulation (DBS) has been approved by the US Food and Drug Administration for treating a range of conditions including Parkinson's disease, epilepsy and obsessive compulsive disorder. Some 180,000 people around the world have brain implants.
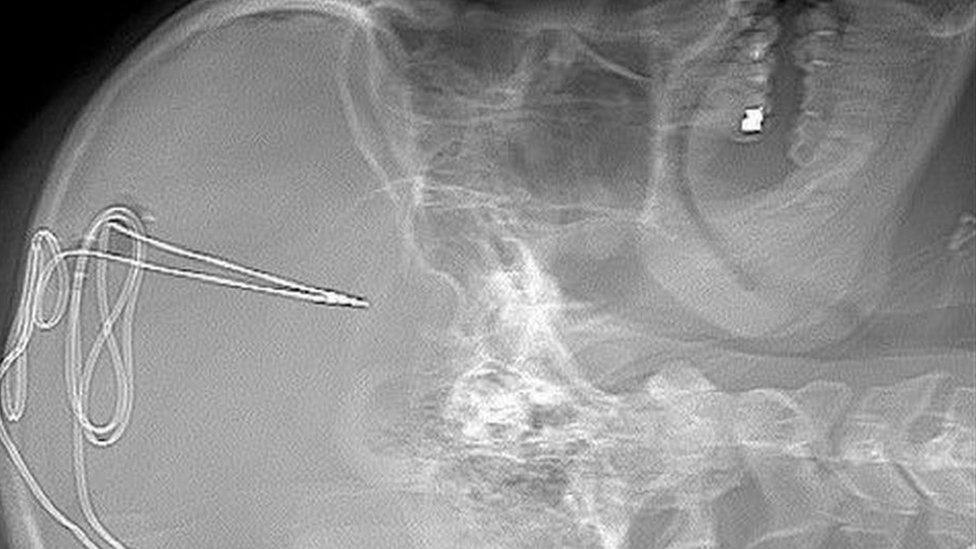
This X-ray shows the brain implant being inserted
This is the first time DBS has been approved for drug addiction and it has been a complex trial, involving many teams, including ethicists, psychologists and many regulators.
Over the next two years the patients will be closely monitored.
Dr Rezai told the BBC: "Addiction is complex, there are a range of social dynamics at play and genetic elements and some individuals will have a lack of access to treatments so their brains will slowly change and they will have more cravings."
"This treatment is for those who have failed every other treatment, whether that is medicine, behavioural therapy, social interventions. It is a very rigorous trial with oversight from ethicists and regulators and many other governing bodies."
He points to figures which suggest overdoses are the main cause of death for under-50s in the US.
"Over half of patients relapse. We need to find solutions because it is a life-threatening situation and something which impacts family and loved ones."

Mr Buckhalter with his family ahead of his operation
West Virginia has the highest age-adjusted rate of drug overdose deaths involving opioids in the US. In 2017 there were 49.6 such deaths per 100,000 people, according to the National Institute on Drug Abuse.
Earlier this year, the UK's Royal Society warned of the ethical dangers of merging machines and humans, and were especially concerned about the plans of technology firms such as Facebook and Elon Musk's Neuralink which have both announced research to develop commercial products.
Neuralink has now applied to start human trials in the US, with electrodes inserted into the brains of patients with paralysis.
And Facebook is supporting research that aims to come up with a headset that can transcribe words at a rate of 100 per minute, just by thinking.
Dr Rezai is sceptical about consumer tech firms getting involved in this area.
"I think it is very good for science and we need more science to advance the field and learn more about the brain. This is not for augmenting humans and that is very important. This is not a consumer technology."
"When it comes to applications, it needs to be heavily regulated. This is not like getting a flu shot or a tattoo. Surgery has inherent risks and is not trivial. It is only for those with chronic disease who have failed all other treatments and are without hope."
- Published10 September 2019

- Published31 July 2019