Batten disease treatment: Parents win battle ahead of court date
- Published

Matthew and Gail Rich say the drug has made a "phenomenal" difference to their daughter, Jessica
Families of children with a rare degenerative disease have won their fight for funding for NHS treatment.
The four children, from Newcastle and Cheshire, have Batten disease, which is incurable and causes seizures, visual impairment and mobility loss.
NHS England confirmed a "deal had been struck" to fund a drug which the families say slows the disease.
Mum Gail Rich, of Tyneside, said "every second, minute, hour of campaigning was worth it".
The National Institute for Health and Care Excellence (NICE) had previously said it could not be certain it was value for money, external. The treatment costs £500,000 per child per year.
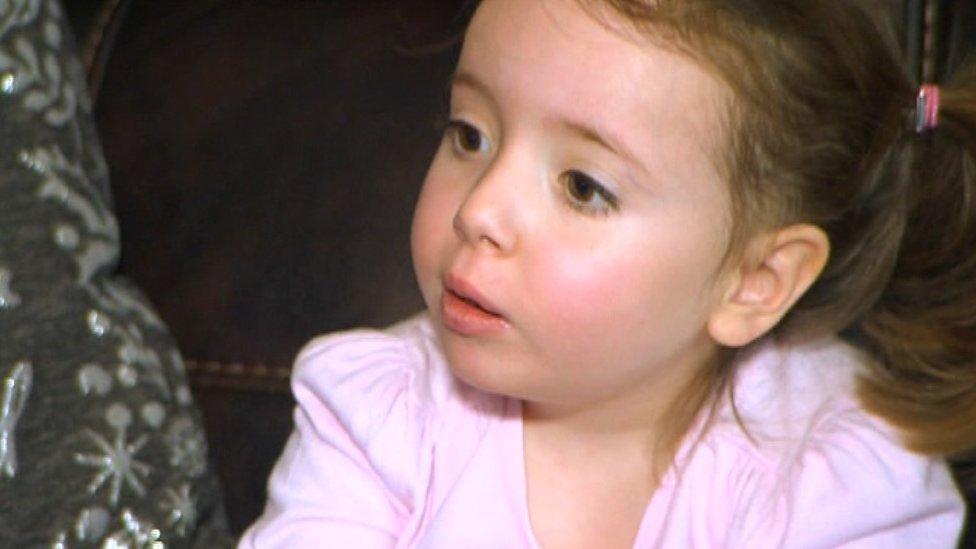
Nicole Rich has had treatment at Great Ormond Street Hospital as part of a drugs trial
But NHS England said manufacturer Biomarin had agreed a "fairer price" for the drug, which is called cerliponase alfa.
Seven-year-old Nicole Rich and her sister Jessica, three, from Throckley, Newcastle, currently receive the drug through a scheme funded by an America pharmaceutical firm.
Their parents, Gail and Matthew, said since taking it, Nicole had shown no further symptoms and Jessica had not developed any.
Speaking to the BBC, Mrs Rich said: "It's phenomenal the difference this treatment makes.
"That's why we were fighting for our daughters and every child in this country who needs it. Every child deserves the same chance.
"It's wonderful news, but we feel very aware it's come too late for some children."
Responding to the decision by NHS England, she had earlier tweeted it was "the day we have been waiting for" and said she and her husband "can't stop crying".
Allow X content?
This article contains content provided by X. We ask for your permission before anything is loaded, as they may be using cookies and other technologies. You may want to read X’s cookie policy, external and privacy policy, external before accepting. To view this content choose ‘accept and continue’.
Lucy and Mike Carroll, from Poynton, also said the treatment had been beneficial for their eight-year-old, Oliver.
His six-year-old sister Amelia has not developed symptoms while taking it.

The parents of Amelia and Ollie Carroll say the treatment has been beneficial
Earlier this year the families won permission for a judicial review to decide whether the decision not to fund treatment was legal. The hearing was scheduled for next month.
A NHS statement said: "This is another concrete step towards ensuring NHS patients with rare conditions get access to important new treatments.
"Coming after extended negotiations, the new deal reached today is a reminder that in order to succeed companies must be flexible and realistic."
Children with the condition live until about 10.
The families' lawyers said, with the treatment they could live to 60.
Related topics
- Published28 August 2019
- Published5 August 2019

- Published22 February 2019

- Published13 March 2018

- Published22 January 2018
