Hope new RSV vaccine could reduce hospital pressure
- Published
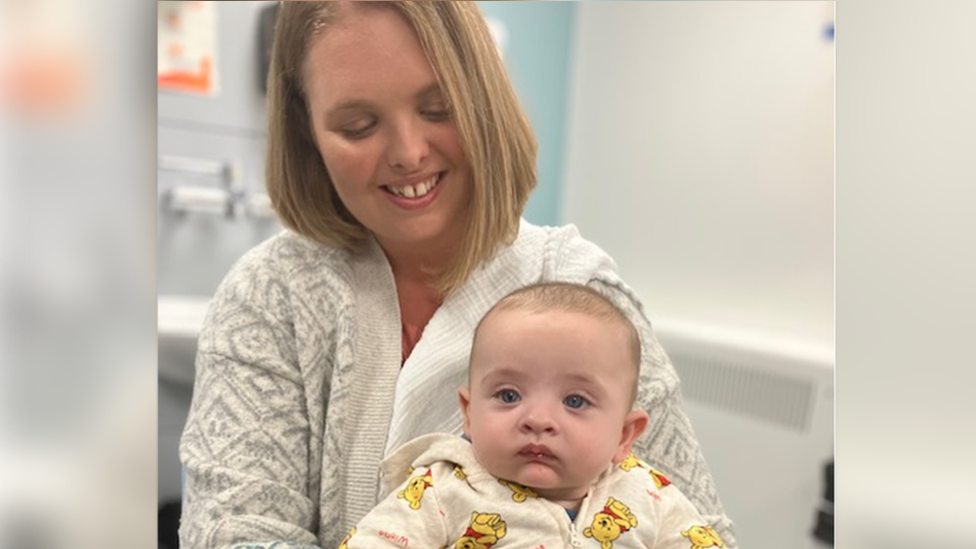
Carly wants to get her five-month-old son Henry vaccinated
Doctors hope a new vaccine for respiratory syncytial virus (RSV) will take pressure off children's hospitals.
Although RSV cases are mild in most patients, it is the main reason children under five end up in hospital.
The Nirsevimab vaccine has been approved but a trial is running in Bristol to explore whether it should be offered to all babies in the UK.
The single antibody shot helps stop infants getting chest infections, such as pneumonia, for about six months.
In a normal winter, RSV mostly causes coughs and colds which clear up in a couple of weeks - but it can be particularly serious in infants under the age of two, causing severe lung problems such as bronchiolitis and pneumonia.
Health officials are concerned many infants now have little natural protection from the usual winter viruses as they were born during the pandemic when lockdowns and restrictions helped suppress their spread.
The BBC understands Bristol Children's Hospital is already busy - with all intensive care beds full on some days in recent weeks.
So if the trial is successful, there are hopes it could reduce demand on paediatric units in the future.
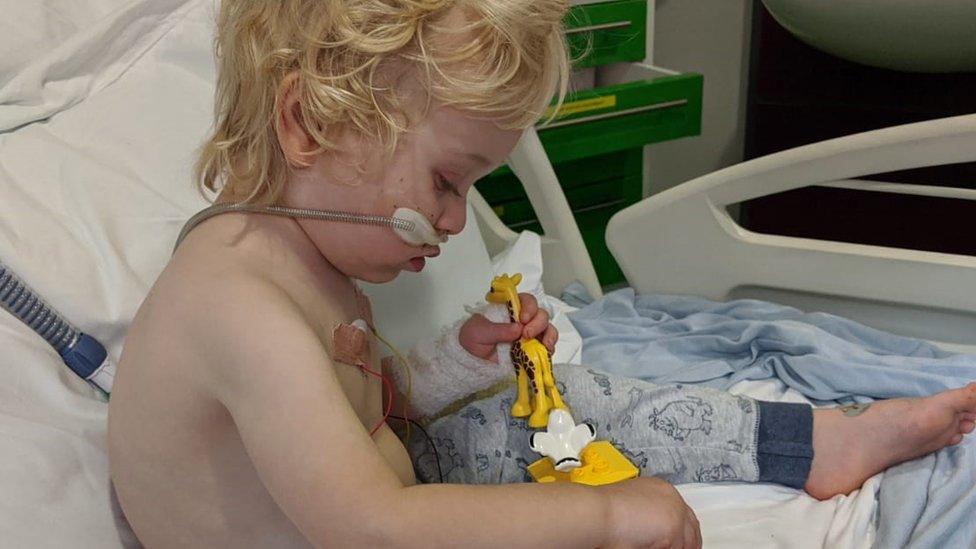
Percy was admitted to Bristol Children's Hospital in 2019
Mum Carly is keen to get her five-month-old son Henry immunised against RSV.
She witnessed first-hand just how dangerous the virus can be when her older son Percy was admitted to Bristol Children's Hospital in 2019.
'Quite scary'
The 32-year-old said: "He was hooked up to monitors. He had high-flow oxygen all the time.
"He couldn't maintain his saturation levels and breathing and it was quite scary. We just had to sit and wait it out."
Her GP practice in Little Stoke is taking part in the clinical trial, which is a collaboration between the National Institute for Health and Care Research and pharmaceutical firms Sanofi and AstraZeneca.
The trial aims to give the vaccine to 20,000 babies across the UK, France and Germany - with a goal of 12,000 jabs in the UK by the end of February 2023.

Dr Nafeesa Arshad said the study was "very safe"
Dr Nafeesa Arshad, based at Concord Medical Centre, says the study is "very safe".
She said: "With over 3,000 babies taking part, we have already had really good safety data and really good evidence to support it.
"But we need these 20,000 more babies to prove its evidence and get it licensed on the NHS."
A spokesperson for Bristol Children's Hospital confirmed it had recently been busy but said it had not yet needed to transfer patients out of the region.

Follow BBC West on Facebook, external, Twitter, external and Instagram, external. Send your story ideas to: bristol@bbc.co.uk , external
- Published10 November 2022
- Published6 October 2022
