Hull family fundraising for US treatment for toddler
- Published

Dan and Daisy Turner hope their son Louie can be treated in the US with a new drug
The family of a two-year-old boy from Hull who has a rare form of cancer say they are facing a race against time to get him the treatment he needs.
Louie was diagnosed with high-risk neuroblastoma last June and given a 50% chance of long-term survival.
His family are now trying to raise thousands of pounds to fly him to America so he can have treatment.
They say Louie now needs a drug called DFMO to give him the best chance of long-term survival.
It is not currently available on the NHS but has been approved in the US.
Louie's mum, Daisy Turner, 28, said: "We first noticed something was wrong when Louie started to go off his food.
"We took him to the doctors and they said if it got worse and if he started vomiting to go to A&E.
"We took him there a couple of days later and they sent us home thinking it was constipation but then we got called back in for more checks and that's when they found the tumour."
His father Dan Turner said the family was "devastated" by the diagnosis.
"If it was low-risk neuroblastoma there is about a 90% chance of long-term survival, but because Louie's is high-risk that dramatically drops and changes to a 50% long-term survival rate," he said.
"The tumour has responded well to chemotherapy and surgery and has reduced in size from 17cm to 0.5cm but, at the moment, the cancer is still active and hasn't shrunk since October."
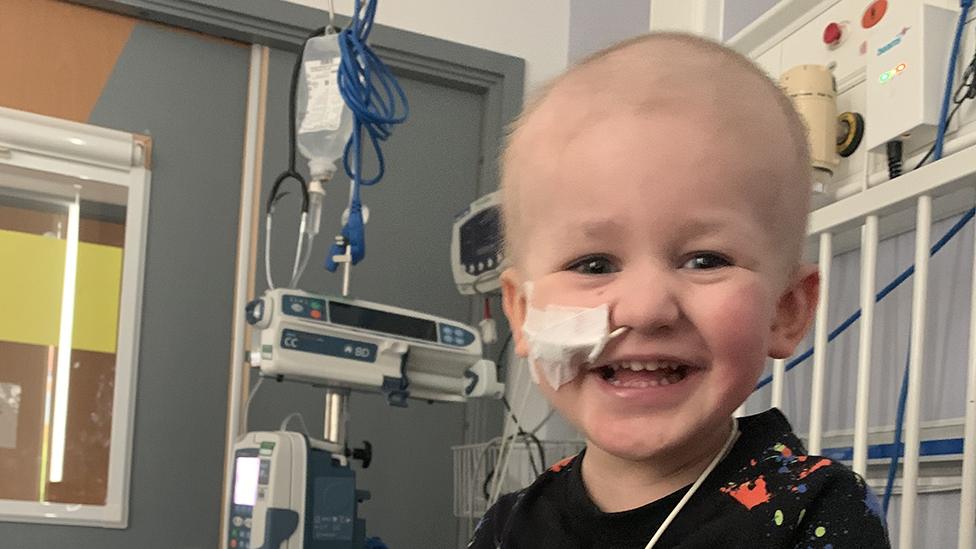
Louie was diagnosed with the rare cancer after he went off his food, his mother said
Mrs Turner said if the tumour began to grow back "there's a chance that doctors here wouldn't be able to do anything to help him".
"We spoke to the doctor in the US recently and she is really positive of long-term survival if Louie gets the DFMO treatment," she said.
"As a parent you just want to give your child everything and do everything to help them."
A spokesperson from the Medicines and Healthcare products Regulatory Agency (MHRA), responsible for regulating medicines in the UK, said: "We are committed to providing safe, timely access to treatment for patients in the UK, and prioritise access to potential life-saving treatments where possible.
"Before DFMO can be approved in the UK to treat neuroblastoma, an application must be submitted to the MHRA with evidence supporting this use. Once received, we conduct a thorough review of the data in the shortest time possible.
"No medicine will be approved unless it meets our stringent standards of safety, quality and effectiveness."

Follow BBC East Yorkshire and Lincolnshire on Facebook, external, X (formerly Twitter), external, and Instagram, external. Send your story ideas to eastyorkslincs.news@bbc.co.uk, external
Related topics
- Published17 December 2020
