Paddington illegal drug sale pharmacist ban upheld
- Published

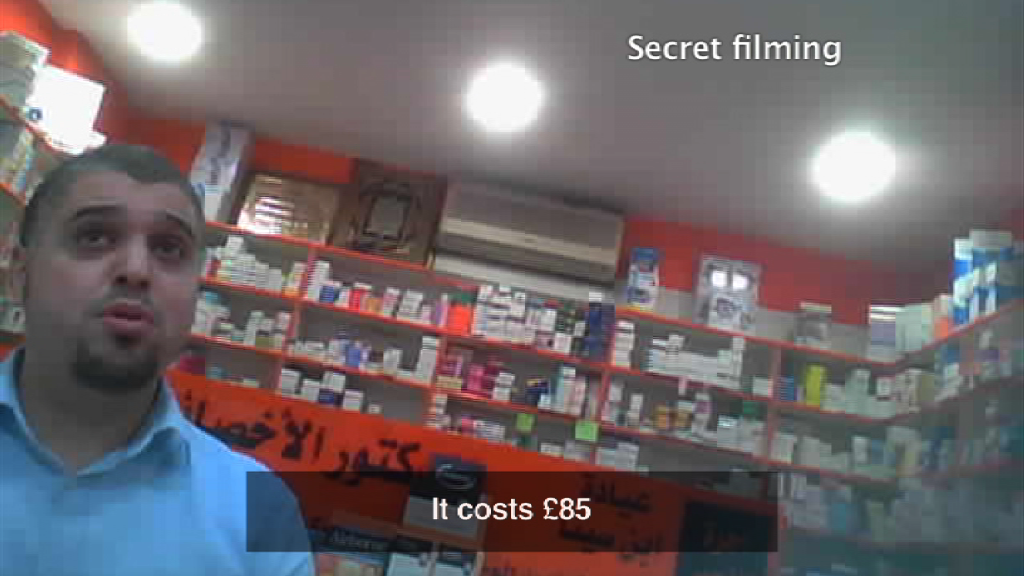
Hussain Jamal Rasool was struck off after a BBC London investigation found pharmacies selling drugs without a prescription
A pharmacist caught illegally selling addictive drugs to undercover reporters "deserved" to be struck off, the High Court has ruled.
Hussain Jamal Rasool was struck off after a BBC London investigation found several west London pharmacies sold drugs without a prescription.
Mr Rasool appealed against the decision arguing it was disproportionate.
The judge said she accepted his actions were "fundamentally incompatible" with his registration as a pharmacist.
'Serious and shocking'
Mr Rasool was removed from the register by a fitness to practise committee set up by the General Pharmaceutical Council.
Mrs Justice Carr, sitting in London, also ruled the committee, was entitled to use Mr Rasool's case to send out an "emphatic" message that in such cases the penalty "must be removal from the register".
Mr Rasool was the superintendent pharmacist at the Al Farabi Pharmacy in Edgware Road, Paddington.
He was on duty on four occasions when undercover reporters with hidden cameras visited the pharmacy between August and November 2012.
The judge said prescription-only medicines, including Amoxicillin, Augmentin, Diazepam and Oramorph, were supplied in exchange for money without a prescription.
Mr Rasool defended his actions by saying the sales were not unlawful as they were all emergency supplies permitted under human medicines regulations.
The committee said the "most serious and shocking" aspect of Mr Rasool's behaviour was the manner in which he was prepared "casually, peremptorily and without any proper inquiry to hand over prescription-only medicines to a stranger upon request in return for cash".
The High Court also rejected Mr Rasool's argument that the committee chairman ought to have stepped down because of apparent bias, and he had not had a fair hearing because of the BBC's failure to disclose certain material to the General Pharmaceutical Council.
As a result of the BBC London investigation at least four pharmacists were further investigated by the General Pharmaceutical Council.
- Published17 January 2013

- Published17 December 2012
- Published17 December 2012
