Nut allergy girl's fear over Emerade adrenalin pen failure
- Published
Beverly Sheppard said her daughter Amber only survived because she was given an adrenalin shot by paramedics
A teenager has said she fears for her life after her adrenalin pen failed when she went into anaphylactic shock.
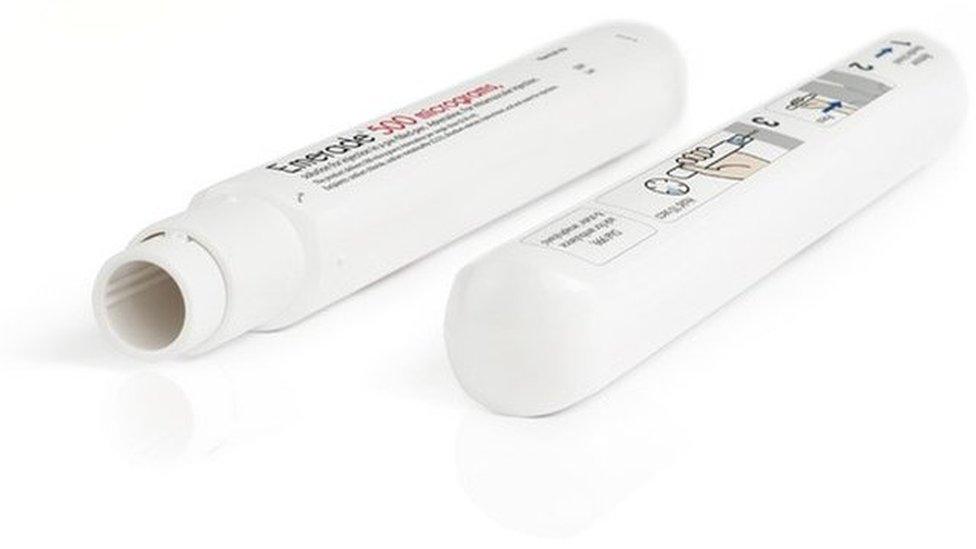
Amber Sheppard, 14, who has a severe nut allergy, was taken to hospital after the needle in her Emerade auto-injector did not fire.
Batches of the pen have since been recalled but because of a shortage of replacements in the UK, patients have been advised to continue using them.
She said: "It was terrifying. I didn't realise they could fail."
Mum Beverly Sheppard described how her daughter started struggling for breath when she went into anaphylactic shock in September.
"It was awful. Her eyes were swollen, there was lots of mucus and she was coughing and wheezing," Mrs Sheppard said.

Amber said when she had used the Emerade in the past it had instantly eased her symptoms
Amber, from Sheffield, used her Emerade 500 auto-injector but it quickly became clear it had not delivered the adrenalin and her symptoms worsened.
"Usually I can actually feel the liquid going into my leg but I couldn't feel it this time and I knew it wasn't working."
There are three brands of adrenaline pens available in the UK - Emerade, EpiPen and Jext - which can all be used to inject adrenaline to someone who is having a severe, life-threatening allergic reaction.
'Frightening time'
A warning about Emerade pens failing to activate was first issued by the Medicines and Healthcare products Regulatory Agency (MHRA) in July and then again in October.
A month later, healthcare professionals were told to stop prescribing the product in the UK after the the regulator issued a recall.
However, because of a shortage of alternative brands patients who had Emerade pens were told to continue using them until they expired but to be informed by the healthcare professional at dispensing of the risks and to carry two pens at all times, external.
But Ms Sheppard said because Amber's pens were in date, they were not aware of the potential faults.
Amber, who is reliant on Emerade because it provides a higher dosage compared to EpiPen and Jext, said she "lost the sense of security" the pen once provided.
She added: "I try to stay positive but sometimes it's difficult when you know that some people have died because of reactions and there's always that fear you might be the next person."

Amber was treated at Sheffield Children's Hospital
Ms Sheppard said: "I try not to worry but that's easier to do when you feel the thing that you carry around to save her is actually going to work and after what happened I don't have that confidence."
Dr Nicola Jay, consultant paediatric Allergist at Sheffield Children's Hospital, said it was a "frightening time" for people with allergies.
"You've got lots of cases being reported in the news about people dying from anaphylaxis.
"At the same time we're hearing reports of auto-injectors not working and also we have then the confounding factor of other auto-injectors not being available."
The MHRA confirmed it had been made aware of 23 suspected activation failures between last July and January in the UK.
The regulator said a full product recall had not taken place because in the UK "there are insufficient supplies of alternative brands to replace all the Emerade pens held by patients".

Dr Nicola Jay said many families were struggling to get hold of auto-injectors
It added: "The risk of not having a pen is much higher than having a pen that may not activate."
Bausch and Lomb, the manufacturers of Emerade, said they did not know how many pens were in the hands of patients in the UK.
The company said there had only been a small number of complaints alleging the pens had not worked - "a proportion of less than 0.003% distributed globally".
It said it was working with the MHRA to conduct a thorough investigation into the reports, adding: "No further supplies of the Emerade will be made available until the activation issue in the manufacturing process has been fully resolved."
Meanwhile, Mylan, the distributors of Epipen, said supply problems were due to manufacturing issues by pharmaceutical company Pfizer and it was working to achieve "a steady state of supply".
The maker of Jext, ALK, said it was increasing production of pens for the whole of Europe, with numbers to increase in the UK from 250,000 to 400,000.
Inside Out (Yorkshire and Lincolnshire) investigates issues around auto-injectors on BBC One at 19:30 GMT on Monday 27 January and can be seen afterwards on BBC iPlayer.

Follow BBC Yorkshire on Facebook, external, Twitter, external and Instagram, external. Send your story ideas to yorkslincs.news@bbc.co.uk, external.
- Published12 July 2019
- Published28 September 2018

- Published25 September 2018
