Sheffield family's world 'fell apart' when son's drug trial ended.
- Published

Harley Bond, pictured with his parents Emma Siddall and Wayne Bond, was diagnosed with Sanfillipo syndrome in 2016
The family of an 11-year-old boy with a rare form of childhood dementia say their world has "fallen apart" since a clinical trial ended abruptly.
Harley Bond was diagnosed with Sanfillipo syndrome in 2016 and began taking part in the drug trial in 2017.
But, when his treatment was withdrawn last year his parents said it "was like being diagnosed all over again".
In a letter, seen by the BBC, US firm Allievex said it lacked "sufficient capital" to continue the trial.
Harley, from Sheffield, was four when he was diagnosed with the disorder, a terminal condition which gradually limits a child's ability to walk, talk and eat.
Doctors said he was not expected to live past 14, but his family was given fresh hope when he started taking the drug Tralesinidase Alfa as part of a trial at London's Great Ormond Street Hospital.
Before he started taking the drug his parents, Emma Siddall and Wayne Bond, said their son's behaviour had become "increasingly erratic".
Mr Bond said: "He completely stopped, as if nobody was there. His speech had totally gone, [he was] very erratic... like a Tasmanian devil."
But when he started treatment, Mr Bond said his condition stabilised and he started using new words.
"He started coming back to us. He was like a new child again."
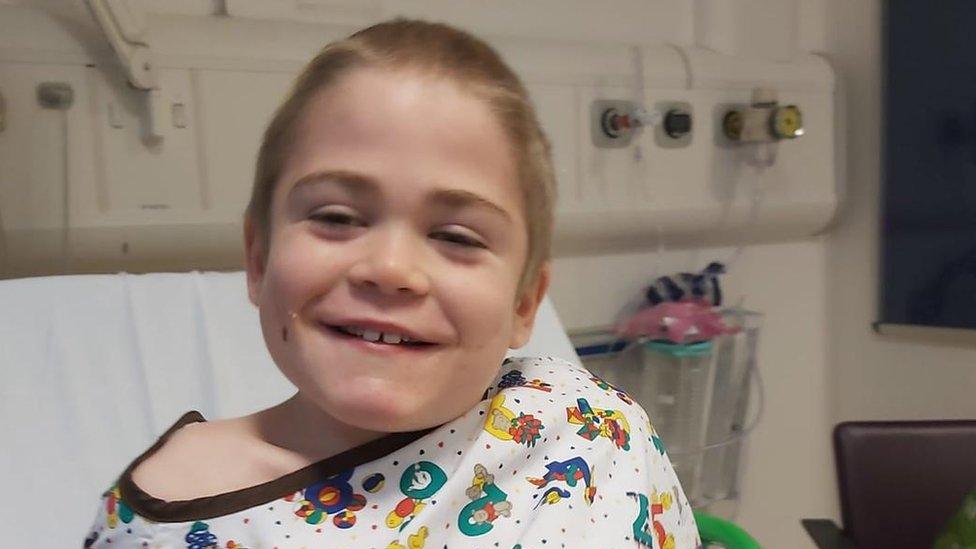
Harley was taking part in a clinical trial of the drug Tralesinidase Alfa
However, despite the drug showing promising results, the trial used to treat Harley and 14 other children was halted in October.
Miss Siddall said: "It was like being diagnosed all over again. Your world just falls apart, you hit rock bottom."
The couple say they are now bracing themselves for changes in Harley's condition.
Mr Bond said: "We know it's coming. Nobody can give us a date when, but the trial was a clean sweep of the toxins and we know it's going to start building up again in his brain and in all his organs."
In the letter the company told clinicians "all clinical activity should be stopped without delay".
It stated the company had been told a new "controlled clinical trial will be necessary to confirm the clinical benefit" of the drug but that it did not have the money to fund it.
Speaking to the Guardian, external, Tom Mathers, the chief executive of Allievex, said pausing the trial had been the "hardest decision I've ever had to make" adding that it was "frustrating because we have a treatment that we know works".
A spokesperson for Great Ormond Street Hospital said: "We know how impossibly hard this situation is for all involved and our efforts are now focused on supporting Harley and his family.
"Our teams will continue to work closely with them, and others at GOSH, who have been impacted by this news, as we explore options and support them through their next steps."

Follow BBC Yorkshire on Facebook, external, X (formerly Twitter), external and Instagram, external. Send your story ideas to yorkslincs.news@bbc.co.uk, external.
- Published26 December 2023
- Published25 August 2022

- Published8 April 2019
