Brexit: EU law change guarantees NI medicine supply
- Published
The European Parliament approved changes to EU law to ensure the supply of medicines from Great Britain to Northern Ireland
Changes to European Union (EU) law aimed at guaranteeing the supply of medicines from Great Britain to Northern Ireland have passed their final stage.
The European Council has ratified the changes, which were passed by the European Parliament last week.
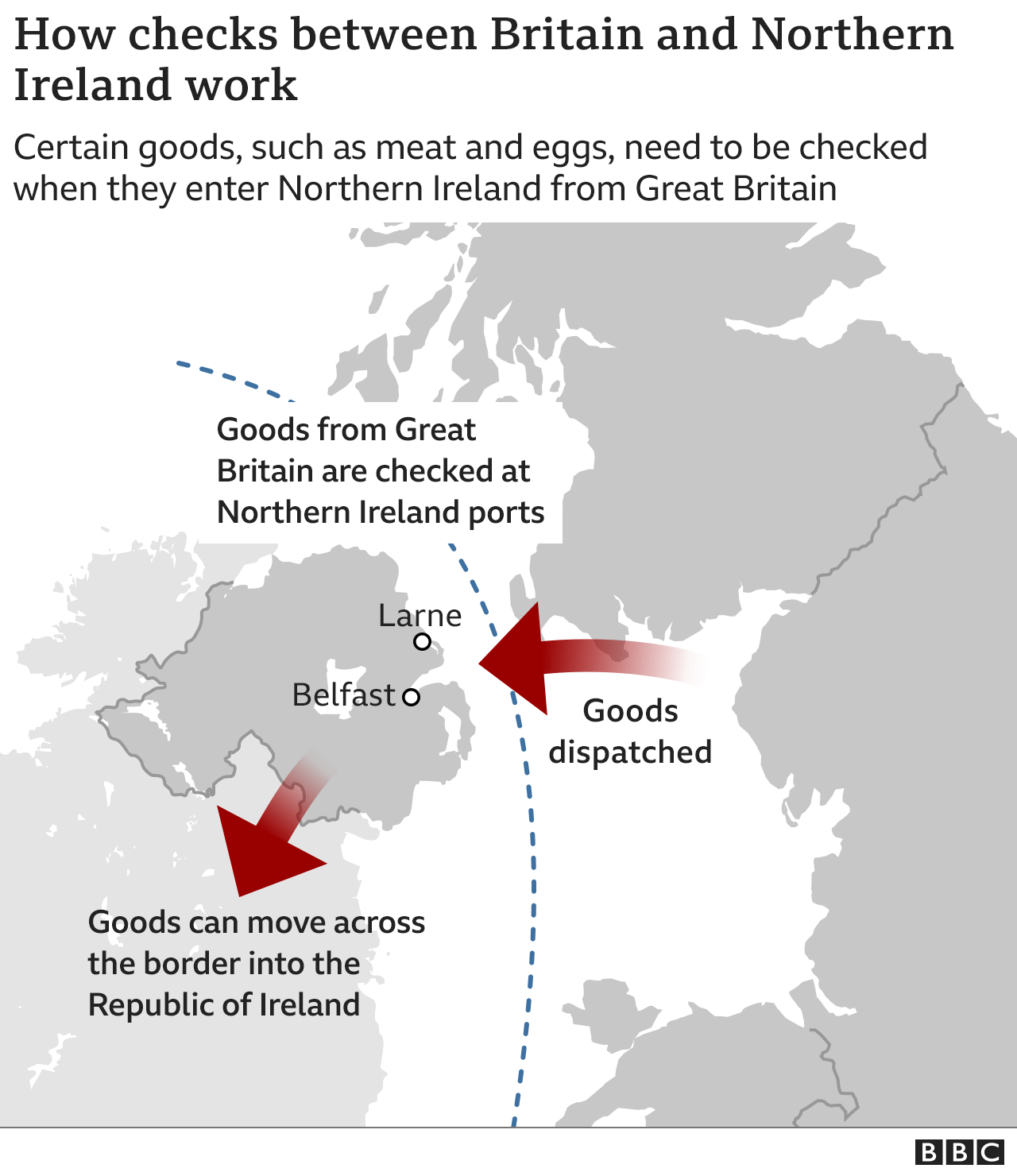
The Northern Ireland Protocol means Northern Ireland is still inside the EU's pharmaceutical regulatory system.
However, it gets most of its medicines from Great Britain, which is not.
This emerged as one of the protocol's major difficulties, with pharmaceutical firms warning it would lead to withdrawal of products.
In December 2021, the EU published proposals which were aimed at creating something close to the pre-Brexit status quo.
The EU's chief negotiator, Maroš Šefčovič, said the changes ensured a continued supply of medicines to Northern Ireland.
"We now have a lasting solution, which was delivered in record time," he said.
"I will continue to work closely with the UK government to ensure predictability, legal certainty and the prosperity of all communities in Northern Ireland."
The changes mean medicines entering Northern Ireland from Great Britain will not need additional labelling or testing, things which would have been required by the protocol in its original form.
Companies located in Great Britain can continue to use the same pack and leaflet for all parts of the UK with no need for NI-specific packaging.
All regulatory functions, like batch testing, will remain wherever they are now in the UK meaning there is no need to relocate any testing facilities from Great Britain to Northern Ireland.
For new medicines, like cancer drugs, any product authorised in the UK can be supplied to Northern Ireland, until the relevant authorisation is also given in the EU.
For generic drugs like paracetamol, the UK regulator can continue to approve drugs for Northern Ireland.
For all types of medicines, no manufacturing authorisation or import licence will be required for bringing medicines into Northern Ireland from the rest of the UK.
The UK government has never formally agreed to the EU proposals but has not objected to them either.
It is understood the UK and EU are no longer negotiating on this issue of human medicines in their efforts to reform the protocol.
However, the UK does want the scope of the changes widened to include veterinary drugs.
Related topics
- Published17 December 2021

- Published18 September 2021

- Published8 April 2022