Cervical screening: Major review of cancer testing delayed
- Published
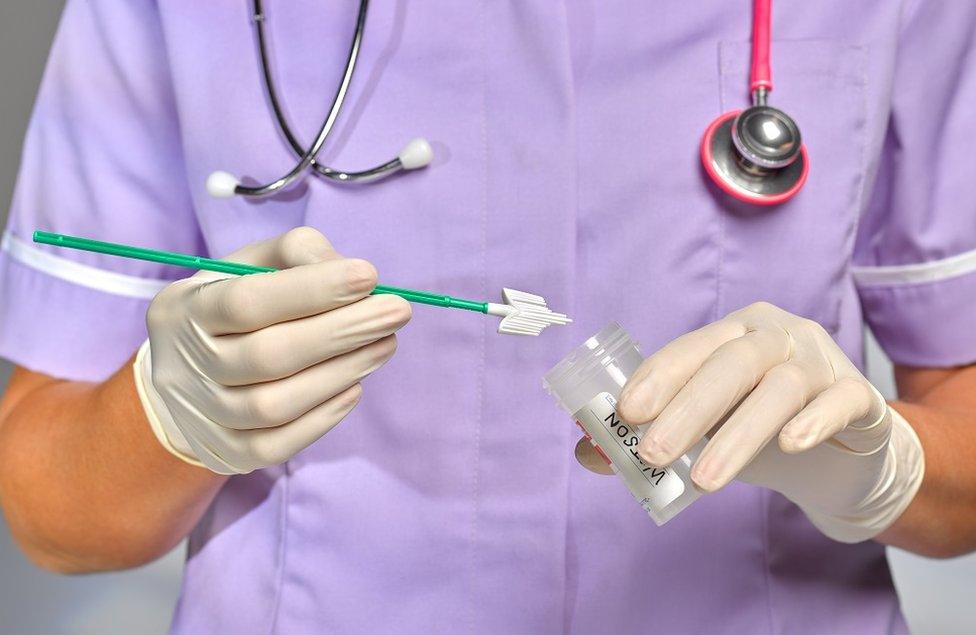
Smear tests, or cervical screening, checks the health of the cervix
A major review of cervical screening in Northern Ireland which was due to start this week has been delayed.
Last month, it emerged smear tests of more than 17,000 women in the Southern Trust would be re-checked as part of a review dating back to 2008.
The Belfast Trust, which was to review the slides, has had its cervical cytology service accreditation suspended, BBC News NI understands.
In a statement, the Belfast Trust said it planned to appeal that decision.
It added it will continue cytology testing as planned and that the Public Health Agency (PHA) has been informed.
The Belfast Trust added that the PHA will continue to monitor and quality review all aspects of their work.
At this stage it is not yet clear whether the Trust intends to include those slides included in the Southern Trust's review or is continuing to screen those slides within its own screening programme.

What is cervical screening?
Cervical screening can not detect cancer, but detecting and treating abnormal cells may help prevent cancer. No screening process is 100% accurate.
The screening looks for the human papillomavirus (HPV) which can cause abnormal cells on the cervix. If HPV is detected a cytology test is used to check for any abnormal cells.
Unlike the rest of the UK and Ireland, Northern Ireland does not have the primary HPV screening system in full operation.
In Northern Ireland, the cervical screening process involves two people - a screener and checker analysing slides under a microscope.

The United Kingdom Accreditation Service (UKAS) is an independent body which assesses the competence of organisations that provide certification, testing, inspection, and calibration services which meet internationally specified standards.
Imposed suspensions are applied when UKAS has identified that the organisation is unable to continue to meet the requirements of the standard for which they hold accreditation.
It can be for a variety of reasons ranging from the performance and availability of staff even broken equipment.
It will also apply if there are failings in the integrity of the organisation's senior management.
BBC News NI understands there may be concern over the performance of screeners at the Belfast Health Trust Laboratory.
It follows a highly critical report into the Southern Health Trust commissioned by the Royal College of Pathologists (RCPath).
It found:
Several cytology staff were "significantly underperforming"
Mechanisms to check their work were flawed
Action taken by management was inadequate over many years
While it is not mandatory for all cervical smear testing laboratories to be accredited, BBC News NI understands that all laboratories involved in the screening programme had been accredited prior to these sanctions being imposed.
'Full faith in the rigorous testing'
In its statement, the Belfast Trust said it wanted to "reassure the public that it continues to have full faith in the rigorous testing carried out within the service".
"We will continue cytology testing as planned and have informed the Public Health Agency (PHA)," the Trust said.
"The PHA undertakes ongoing monitoring and quality review of all aspects of the work of the cervical cytology screening programme and based on this are assured that the work of the cervical cytology service is to an appropriate standard."
UKAS confirmed to BBC News NI that accreditation for cervical cytology within the Belfast Health Trust had been removed on 31 October.
The same measure had been applied to the Southern Health Trust, as reported by BBC News NI last month.
When an accreditation is suspended or withdrawn, the organisation can no longer provide accredited services and must inform its existing and prospective customers of this status.
Related topics
- Published26 October 2023

- Published9 October 2023