Relax rules on research into illegal drugs, say advisers
- Published
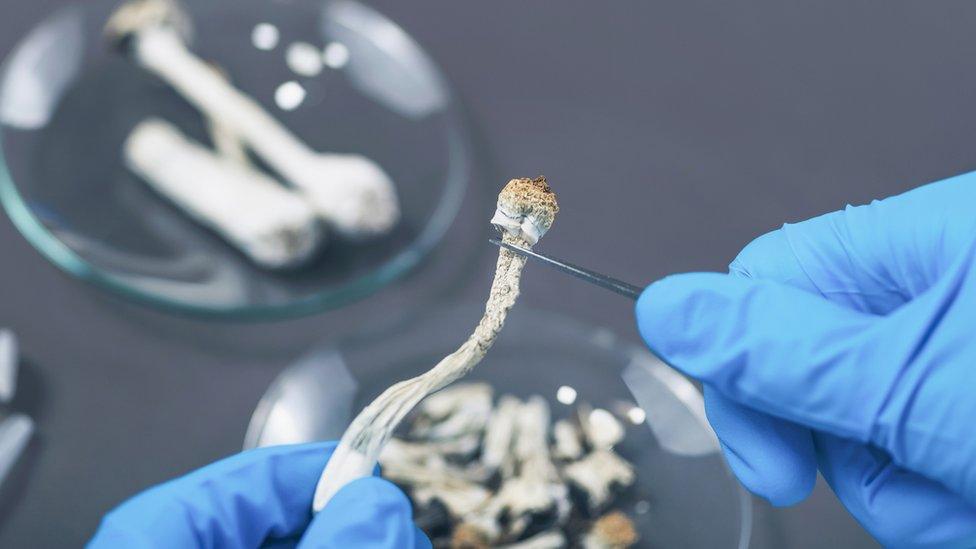
Researchers have found psilocybin, a drug found in mushrooms, could help treat severe depression
Independent government advisers have called for rule changes to allow more research into therapeutic uses of illegal drugs, including psychedelics.
The Advisory Council on the Misuse of Drugs (ACMD), external proposes easing controls on trials using Schedule 1 drugs - which include opium and LSD.
Recent studies have found psychedelics could help treat severe depression.
MP Charlotte Nichols said further research could lead to "transformative mental health treatments".
Ms Nichols has previously spoken in Parliament about a need for research into the potential medicinal uses of hallucinogenic mushrooms to treat her PTSD.
The Labour MP described her condition as "living hell".
Ms Nichols, who chairs the Centre for Evidence Based Drug Policy (CEBDP) forum, said the report was "an important milestone on the road" towards better treatment.
"We have high hopes that the government will now urgently work to break down barriers to research into LSD, psilocybin, MDMA and other drugs with the potential to be transformative treatments for anxiety, severe depression and PTSD," she said.
Research into therapeutic uses of cannabis, also a Schedule 1 drug, have led to two new drugs coming to market in the UK - Epidyolex for treating seizures and Sativex which can alleviate neuropathic pain.
There have also been recent successes in using a drug based on a compound in hallucinogenic mushrooms to improve the symptoms of severe depression.
Psilocybin appeared to free up the brains of people with severe depression in a way that other antidepressants did not.
The CEBDP has campaigned for the drug to be taken out of Schedule 1 and reclassified so it can be accessed by more patients, as it is in Australia and Canada.
'Right balance'
The government commissioned the ACMD last December to look into barriers to research around controlled drugs.
The ACMD found the process of applying for a licence to use Schedule 1 drugs like psilocybin in scientific trials could take a year.
Tough rules and extensive paperwork mean only major, well-funded studies can carry out clinical trials.
If granted, licences are valid only for three months - which is often insufficient for organising shipments of drugs and managing paperwork.
Relaxing these rules would open up research, allowing more academic and medical studies, the ACMD said.
It also said a new regime could help find the safest and best therapeutic uses of Schedule 1 drugs, external.
A Home Office spokesperson said: "We welcome the ACMD's report and will consider the findings carefully."
Chris Philp, the Home Office minister responsible for drug policy, has previously said "ensuring that the UK is a world-leading jurisdiction for pharmaceutical, clinical and other medical research is a top government priority".
But, in a letter to the ACMD before the report was published, Mr Philp said the government must "maintain the right balance between access for legitimate research whilst minimising the risk of harm, diversion, and misuse of controlled drugs."
Related topics
- Published1 July 2018
- Published18 May 2023
- Published18 May 2023
- Published27 March 2023