Cancer drug Kadcyla approved for use by NHS Scotland
- Published

Lesley Stephen, Lesley Graham, Anne Maclean-Chnag and Alison Tait led a campaign to have Kadcyla approved
A breast cancer drug which is being withdrawn in England because it is too expensive is being made available on the NHS in Scotland.
Kadcyla is used to treat an aggressive, advanced type of breast cancer known as HER2 positive.
The Scottish Medicines Consortium (SMC) said the drug would enable patients to spend "more time with their families and in some cases return to work".
Scottish health secretary Shona Robison said the SMC made a "good decision".
She added that it was expected to benefit about 100 women a year north of the border.
The move was welcomed by campaigners and charities which pointed out that Scotland is now one of 18 countries, including France and Germany, which offers the drug.
'Inspirational' campaigners
Patients in England can get the treatment through the Cancer Drugs Fund, but it has not been made routinely available by the National Institute for Health and Care Excellence (Nice).
Late last year Nice published final draft guidance saying Kadcyla is not set at an affordable price.
In Scotland, Breast Cancer Now said they worked alongside four "inspirational" women - all with incurable secondary breast cancer - who led the "Unlock Kadcyla" campaign.
More than 13,000 people signed a petition which was presented to the SMC and drugs company Roche.
The charity's director for Scotland, Mary Allison, said: "This decision will transform treatment options for women with HER2 positive secondary breast cancer in Scotland."
She added: "We are pleased that the SMC and Roche have worked together to unlock this revolutionary drug. We hope this will be just the start of improved access to breast cancer medicines in Scotland.
"Both the Scottish government and Breast Cancer Now share the same vision of making sure that by 2050 everyone who develops breast cancer will live.
"If we are to achieve this, we'll need to ensure that patients in Scotland are able to access the best possible treatments - and today is a real step forward for women with HER2 positive disease."

What is Kadcyla and what does it do?
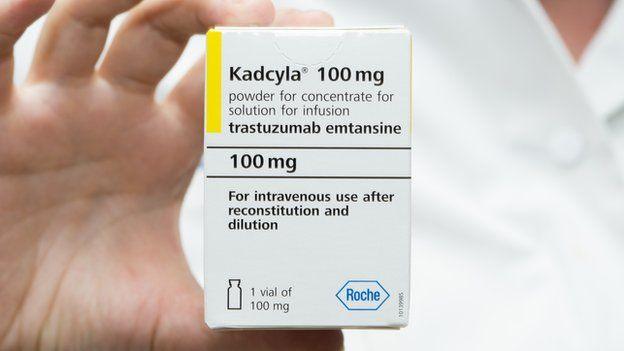
Kadcyla, external is used to treat breast cancer which has spread to other parts of the body.
It is made from two cancer-fighting drugs, the monoclonal antibody trastuzumab (Herceptin) and the chemotherapy drug emtansine.
The treatment is only right for patients with a certain type of tumour - one that is HER2-positive.
Kadcyla has been made to deliver chemotherapy into HER2-positive cancer cells and kill them.
It is designed to cause less harm to normal cells.
However, there may be side effects to the liver, heart and lungs.
BBC health correspondent Nick Triggle wrote about the drug in 2014, spelling out how its high cost was causing a funding conundrum.

One of the women involved in the campaign, Alison Tait, said breast cancer patients and their families had been given a lifeline.
"I couldn't be happier," she said.
The single mother from Edinburgh added: "I'm not thinking about me today. I'm thinking about my daughter Ellen. I'm glad that this drug, and the extra time it gives, will be available to all women who need it.
"For my family it means that Ellen and I have the best chance of sharing those big life moments together - that we can share more of life."
Nicolas White, head of Scotland at Breast Cancer Care, hailed the move as a "landmark decision".
He said: "Kadcyla can mean an extra six months with loved ones to make countless more precious memories - that time is priceless.
"These women already live with extreme uncertainty every day, and worries about not being able to access the drugs they need to live longer only add to their anxiety. Knowing that Kadcyla is now on the table offers a real glimmer of hope.
"We now need to keep up the momentum in approving life-extending drugs for all types of incurable breast cancer. The best possible treatments must be available to all, yet too many are still kept out of reach.
"This is just one part of a complex puzzle we must solve to ensure nobody is left receiving the second-rate care so many currently experience."
- Published6 March 2017

- Published16 January 2017