Family of brain tumour patient Caroline Burns in cannabis plea
- Published

Caroline and Gary Burns have cashed in pensions and spent their savings on cannabis treatment
The husband of a brain tumour patient has written to the Home Secretary after spending £1,250 a month on medicinal cannabis.
Gary Burns says cannabis is the only treatment that has had a "positive effect" on his wife, Caroline's tumour.
But he has to buy it illegally from Canada as their application for a UK licence for the treatment was rejected.
The Home Office said specialist doctors would be able to legally prescribe cannabis-based products from autumn.
Mr Javid announced in July that products that meet safety and quality standards would be made legal for patients with "exceptional clinical need".
It followed a high-profile campaign led by the parents of two severely epileptic children who said cannabis oil controlled their seizures.
The Scottish government said it was important the NHS in Scotland was involved in drawing up guidelines on the matter.
Speaking to Kaye Adams on BBC Radio Scotland, Mr Burns accused the Home Office of "dragging its feet" in relaxing laws governing access to cannabis-derived medicines.
"It just seems that the Home Secretary pushed out this because there was a big uproar in the news with the two young boys, and then what's happened from there is it's slowed right down," he said.
"Unfortunately for us, we can't have it slowing down like that. We need it to be as fast as possible."
He said his wife was given a prognosis of about 18 months when she was first diagnosed with the tumour about five years ago.
Two years later, when she was told she had three months to live, Mrs Burns rejected chemotherapy and started cannabis treatment.
It has come at a considerable cost to the family, from Glasgow, who have spent in excess of £40,000 over more than three years.
"Family and friends help you do fundraisers, we've cashed in pensions, we've spent all our savings," Mr Burns said.
"Family and friends have given money - just for someone to live their life, it's not right."
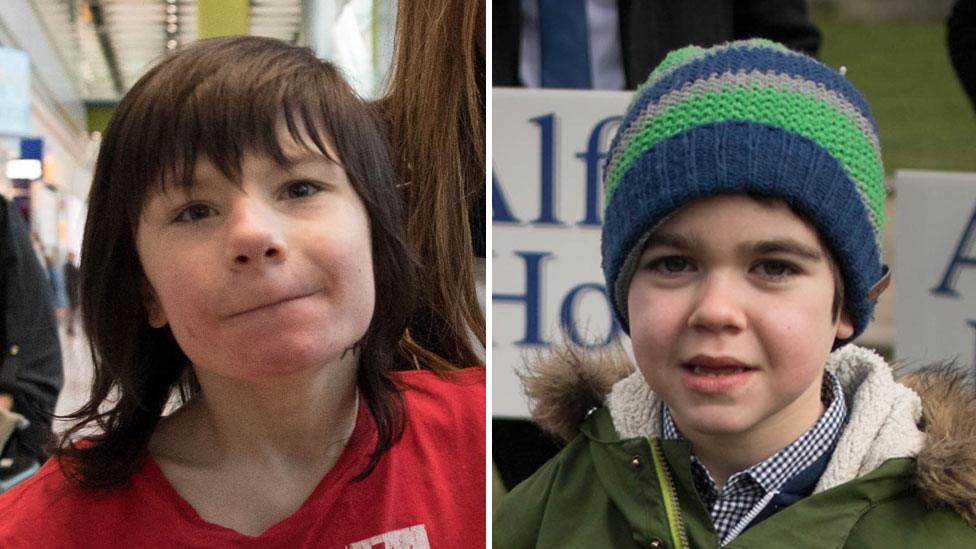
Billy Caldwell and Alfie Dingley were granted licences to allow them access to cannabis oil
Following the cases of Billy Caldwell and Alfie Dingley, the couple applied for a licence to obtain cannabis oil for Mrs Burns.
Both boys received the treatment after being granted a licence by the Home Office.
But Mr Burns said that when they tried to apply for a licence, doctors refused to refer them to the expert panel.
He said that, since starting the cannabis treatment, Mrs Burns' tumour has been downgraded from grade four to grade three.
"They've tried the chemo, they've tried the radiotherapy," he said. "It didn't work so at that point we stopped. [The tumour] was actively, progressively getting bigger. That's why they said it roughly three months until end of life.
"At that point we decided to [start cannabis treatment]. Since we've done that, it has dropped. One of the last MRIs we got about six months ago, they did say it had dropped about 26% overall. Not from operation, just from shrinkage."
'Swift action'
But he is angry that the family are having to fight for the treatment which he believes is helping his wife.
"Whether Caroline has got five weeks, five days, five years, five months, we shouldn't be fighting to get this," he said.
"We should be living our life as happy as we can. We shouldn't be spending most of our time fighting to get something that should, rightly, be out there."
A Home Office spokesman said they "completely sympathise" with families "facing desperate situations as they try to find treatment".
"In July the Home Secretary committed to swift action on behalf of those whose medical conditions could potentially be eased by cannabis-based products and we have announced that cannabis-based products for medicinal use will be available for specialist doctors to prescribe legally from the autumn.
"Any proposed course of treatment with cannabis-based medicine must be clinically led"
A Scottish government spokeswoman said: "The expert panel was established in June to advise UK ministers on medicinal cannabis licence applications made by senior clinicians on behalf of patients.
"The decision on whether to make an application to the panel is purely one for the treating clinician.
"However, it is important the NHS in Scotland is involved in the development of clinical guidelines in this area to support doctors and make sure prescribed products are safe and effective, including for children."
- Published26 July 2018

- Published26 July 2018
