Mesh expert failed to declare £100,000 funding
- Published

Prof Mohamed Abdel-fattah has now corrected his funding disclosure
A high-profile academic has admitted he failed to declare £100,000 from the manufacturer making one of the types of vaginal mesh implant he was assessing.
Aberdeen University's Prof Mohamed Abdel-fattah has published a correction to his original research paper almost seven years on.
The change follows a complaint to the General Medical Council about him.
The doctor who made the complaint said it was "shocking" the funding had not been declared at the time.
Prof Abdel-fattah denied that there was any link between the funding and the study.
Mesh implants are used by surgeons to treat conditions which some women suffer after childbirth.
The synthetic mesh is used to repair damaged or weakened tissue in the woman's body.
Many women have been left suffering chronic pain following the surgery.
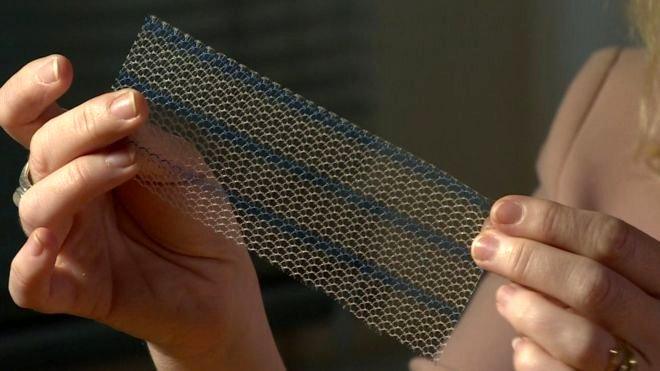
About 40,000 women across the UK have had the transobturator mesh tape procedure
Tens of thousands of women across the UK have been given them over the past 20 years.
However, the use of mesh in Scotland was suspended in all but "exceptional circumstances" in 2014 after it emerged some women had suffered painful side-effects.
Prof Abdel-fattah was the lead clinician on a 2012 study that concluded that no patients were suffering thigh pain three years after their surgery.
His study had compared two types of "transobturator" mesh tapes.
In his original declaration Prof Abdel-fattah said funding, about £65,000, came from The Henry Smith Charity - but he did not mention the £100,000 received from the manufacturer of one of the devices used in the study, Coloplast.
Figures show around 40,000 women across the UK have had the transobturator mesh procedure.
It was the most commonly performed bladder surgery in Scotland and is also used in England.
A complaint was made to the GMC in 2018 with allegations of research misconduct at various levels.
Prof Abdel-fattah has now published a correction to his 2012 report, external admitting funding from the manufacturer but denied any link to "any aspect of the study at any time from design to publication".
He said the research grant from Coloplast was "inadvertently omitted" from the original disclosure.
The correction also contains a link to Prof Abdel-fattah's own declaration of interests. These include receiving money from a number of mesh manufacturers for being a consultant and trainer for mesh manufacturers including Ethicon and Coloplast.


What is vaginal mesh?
It is an implant used to treat incontinence and repair pelvic organ prolapse.
The mesh - which can be synthetic or biological - can support the vaginal wall or internal organs.
How many women have had the implants?
It is estimated more than 100,000 women have had a mesh fitted in the UK.
About 27,000 were in the past 10 years - but 211 women have had it removed.
Why are some women angry?
Some say the implant has caused them severe pain by cutting into internal tissue.
More than 800 women are taking legal action against the NHS and mesh manufacturers over the mesh.
However, the vast majority have suffered no side-effects.
What do health bosses say?
In Scotland, health boards were ordered to stop the use of implants last year.
Health guidelines in England say women should only be offered mesh surgery as a last resort, or for research purposes.
What are the alternatives to mesh?
Physiotherapy and traditional surgery are also an option, depending on the severity of the problem.

'Highly influential'

Dr Wael Agur made the complaint to the GMC
Dr Wael Agur, a consultant urogynaecologist and expert in mesh, made the original complaint to the GMC.
He said Prof Abdel-fattah's study was "highly influential".
Dr Agur said it was "shocking" that the funding from Coloplast had not been declared at the time.
He said: "This study had reassured us seven years ago that the use of mesh is safe - so we continued using it.
"Now we find out that the researchers had received £100,000 from the mesh manufacturers. Why was this money never declared at the time?
"The women trusted us to do the right thing and we thought at the time that we were doing the right thing."

'Life ruined by mesh'

Lorna Farrell is in constant pain and needs a wheelchair to get about
Lorna Farrell had transobturator mesh fitted in 2008.
She says she now lives in constant pain and uses a wheelchair to get about.
She says her life has been "ruined" by mesh.
Lorna told BBC Scotland: "We need to have research into it but how can it be that the research is part-funded by the manufacturers?
"If they want to fund research why don't they speak to those who have been damaged by what they have done."

Restricted use
The GMC said it could not comment on individual cases.
But the BBC understands a failure to declare funding from a pharmaceutical company in a research study could potentially raise an allegation about the fitness to practise of a doctor.
A statement from Aberdeen University said: "Prof Abdel-fattah has been informed by the GMC that they looked into a complaint made against him but the investigation has now been closed as of December 2018.
"Neither Prof Abdel-fattah nor the university are aware of any further ongoing investigation."
The statement added: "The GMC did not ask him to make the amendment to the paper, he did so of his own volition in the interests of increased transparency.
"The amendment clearly stated that Coloplast had no input to the study in any form and at any stage."
The university pointed out that transobturator tapes had been the most common procedure of this sort worldwide since at least 2009.
The use of mesh in Scotland has been controversial.
It has been used hundreds of times since the partial suspension in 2014.
Last year, transabdominal mesh was cited as an underlying factor in the death of a Scottish woman and the Scottish government called for an immediate halt to its use until a new "restricted use protocol" is drawn up.
NHS England has also recently curbed the use of mesh on safety grounds, although it is still available as a treatment of last resort for some.
- Published12 September 2018

- Published26 October 2018