'Life-changing' cystic fibrosis drug deal for Scotland is welcomed
- Published

SNP MP Marion Fellows with granddaughter Saoirse, who was diagnosed when she was three weeks old
Families have welcomed a deal which will make two "life-changing" cystic fibrosis drugs available to eligible patients in Scotland.
A five-year agreement has been reached between the Scottish government and pharmaceutical company Vertex over the use of Orkambi and Symkevi.
The drugs improve lung health but were rejected for NHS use last month because they were not deemed cost-effective.
The medication normally costs £100,000 per patient, per year.
The Scottish government said it had secured a "confidential discount" from the manufacturer.
Patients in other parts of the UK are still not able to access the treatment, with campaigners calling for an end to the "postcode lottery".
'Never let up'
SNP MP for Motherwell and Wishaw Marion Fellows, whose three-year-old granddaughter Saoirse has cystic fibrosis, said it was a "big moment" for patients and their families, who had been "relentless and inspiring" in their campaign.
Ms Fellows said: "I'm so glad that so many families across Scotland will benefit from these new drugs. Hundreds will be able to lead longer and fuller lives thanks to this decision.
"The Scottish government never let up in discussions and is leading the way in cystic fibrosis treatment.
"But cystic fibrosis knows no borders. The UK government must follow the Scottish government's lead and make it available to sufferers in England also."
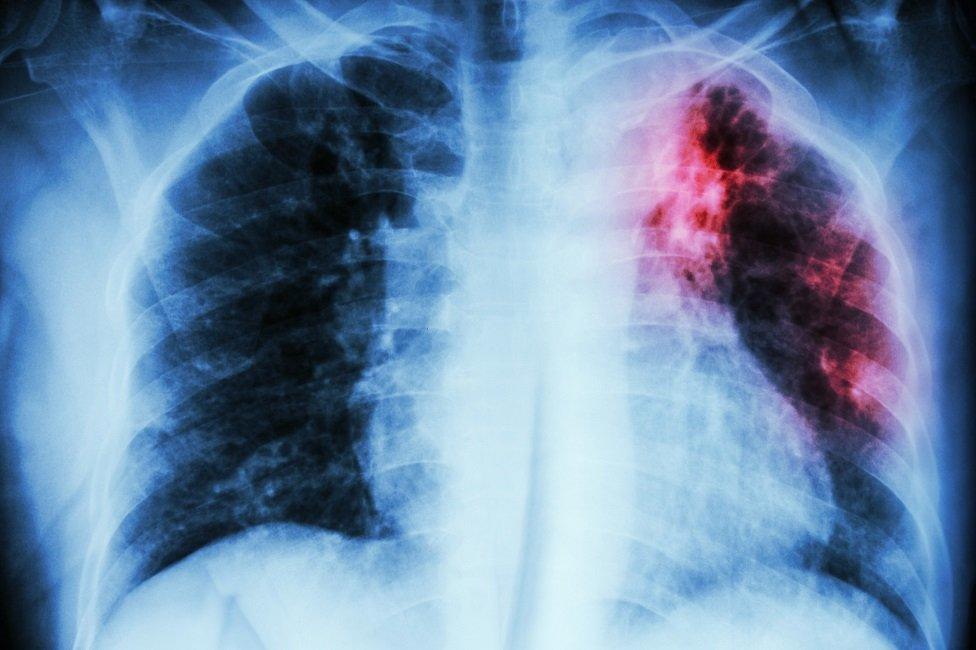
The drugs can reduce hospital admissions by stabilising lung health
Cystic fibrosis is a life-shortening genetic condition that causes fatal lung damage, and affects about 10,400 people in the UK - around 900 of them in Scotland.
Only around half of those with the condition live to the age of 40.
NHS Scotland estimates that one in 24 Scots have a CFTR mutation which, if carried by both parents, would lead to a child being born with cystic fibrosis.
Glasgow mum Gail Gilmour, whose son has cystic fibrosis, tweeted that there were "no words adequate" to thank those who helped secure the deal for Scottish patients.
Allow X content?
This article contains content provided by X. We ask for your permission before anything is loaded, as they may be using cookies and other technologies. You may want to read X’s cookie policy, external and privacy policy, external before accepting. To view this content choose ‘accept and continue’.

Others have said that they hoped it would lead to progress in negotiations for England, Wales and Northern Ireland.
Earlier this year campaigners appealed to the UK government to use its powers to break a deadlock in making Orkambi available on the NHS in England.
Vertex refused a £500m offer for the drug over five years - which was described as the "largest commitment" NHS England had ever made.
Those affected want other drug firms to be asked to make a cheaper version.
The UK government's Department of Health said its approach was still to urge Vertex to accept NHS England's "generous offer".
'Landmark moment'
The Scottish Medicines Consortium (SMC) had rejected the routine use of the drug in August, saying there were uncertainties about the long-term health benefits of Orkambi and Symkevi in relation to their costs.
David Ramsden, chief executive of the Cystic Fibrosis Trust, said the deal was a "landmark moment" for the hundreds of people with CF and their families across Scotland.
"This breakthrough is a victory for their perseverance and enduring hope. It means 350 eligible people living in Scotland will have access to drugs that stabilise their lung health and reduce the need for hospital admissions."
He said the trust would continue to campaign for access to the drugs in England, Wales and Northern Ireland, adding: "Scotland's success must now be replicated across the UK without further damaging delay."

What are Orkambi and Symkevi?
Since 2015, the drug Orkambi has been licensed to treat cystic fibrosis in patients from two-year-olds to adults, who have a specific genetic mutation known as F508del.
It was not available on the NHS, except for certain people on compassionate grounds.
Symkevi is used to treat the same mutation in patients age 12 and older.
The mutation causes the production of an abnormal protein that disrupts how water and chloride are transported in the body.
Orkambi has been shown in clinical trials to improve lung function and respiratory symptoms in people with cystic fibrosis.
It is the first of a string of drugs that have been developed, with newer ones expected to be even more effective.

Scotland's Health Secretary Jeane Freeman said the "fantastic news" would allow cystic fibrosis patients to "live fuller lives for longer".
"The agreement has been reached after extensive discussions between the Scottish government and Vertex Pharmaceuticals and means the medicines will now be made available to patients on the NHS in Scotland, subject to a confidential discount," she added.
Vertex has also committed to collecting real-time data on the medicines to support any future submissions to the SMC.
Ludovic Fenaux, Vertex senior vice president, said: "We would like to thank the Scottish authorities for their partnership and the collaborative and flexible way that we have worked together to find this access solution.
"It means that approximately 400 eligible cystic fibrosis patients in Scotland now have access to Orkambi and Symkevi."
Some Scottish patients have previously had access to Orkambi and Symkevi through the Peer Approved Clinical System Tier 2 (PACS Tier 2), which allows doctors to apply for access on behalf of individual patients.
A spokesperson for SMC said the pharmaceutical industry had responsibility for bringing their medicines to market at a fair price.
"The SMC members make these difficult decisions based on extensive clinical experience and taking all of the available evidence into account, including that provided by patient groups. We look forward to receiving new evidence from Vertex on the impact of these medicines following the agreement they have reached with the Scottish government."
- Published12 August 2019

- Published4 February 2019
- Published15 August 2018
