Covid in Scotland: When will I be vaccinated against the virus?
- Published

The coronavirus vaccination campaign is now well under way in Scotland, and we have a good idea of how plans to deploy the vaccine are shaping up.
The Scottish government has said the first two priority groups - including care home staff and residents, frontline health workers and over-80s in the community - would have had their first jab by 5 February.
Those aged 70 and over will be vaccinated by mid-February.
People aged over 65 and those who are clinically extremely vulnerable will be vaccinated by early March.
This covers the first five of the nine priority groups, which is just over 1.4 million people.
Three vaccines have now been approved by UK regulators - those developed by Pfizer-BioNTech, Oxford-AstraZeneca and Moderna.
They have been purchased by the UK government and Scotland will get a share of them, based on its population.
How will Covid vaccines be rolled out in Scotland?
Current modelling indicates that Scotland will have the capacity to deliver about 400,000 vaccinations a week from the end of February.
GP practices, health centres and local clinics have already been delivering vaccinations.
From Monday 1 February, mass vaccination centres, including Edinburgh International Conference Centre (EICC) and Aberdeen's P&J LIVE at TECA, will be in operation.
The Scottish government said the EICC will have capacity to vaccinate more than 21,000 people a week at 45 stations.
The centre in Aberdeen will start with 20 booths, vaccinating around 6000 people weekly.
The NHS Louisa Jordan mass vaccination centre in Glasgow has been operating since 8 December, carrying out 1,000 to 5,000 vaccinations daily. The facility has the capacity to move to 10,000 per day.
Other large centres will operate at Ravenscraig Sports Facility in Motherwell and Queen Margaret University in Musselburgh - as well as at leisure centres and town halls.
Centres were planning initially to be opened seven days from 08:00 to 22:00, but that could be extended.
It won't be practical for everyone in Scotland to travel to receive their vaccine.
So, in some remote communities, a mobile vaccination unit will be set up to offer immunisations.
In this case, public health officials say they will likely make one trip to the area and vaccinate everyone in the priority group at once - which might mean someone lower down the priority list will get vaccinated earlier than they would had they lived in an urban area.
One plan public health officials are looking at is using mobile libraries to deliver vaccines in remote communities.
What about everyone else?
The hope is to have people aged 50 and over vaccinated by the start of May.
The number of injections vaccinators will administer will be dependent on supply - so there might not be a smooth increase in how many people have received their immunisation.
Quite quickly it's expected the number of injections given to begin increasing.
That's because initially things were very focused on getting those in care homes vaccinated first.
Because the vaccine had to be taken to the homes, it has taken a longer time to get through than if the same number of people had been able to turn up at a mass vaccination site.
By the autumn everyone in Scotland over the age of 18 should have been offered one of the Covid vaccinations. That's 4.4 million people.
But this will depend entirely on supplies - and just now, public health experts say they only know how many doses they can expect until June.
From March, a second stream of second doses of the vaccinations will also be getting under way as the latest guidance is that the second jab should be received up to 12 weeks after the first.
There are no plans to make vaccinations mandatory.
Which vaccines will be available?
The UK government is ordering vaccines on behalf of the four nations, and the supply is then be divided up between Scotland, England, Wales and Northern Ireland on the basis of population.
Scotland's share of the UK's vaccine supply equates to its 8.2% share of the UK population.
The UK government has secured early access to more than 400 million doses of experimental vaccines.
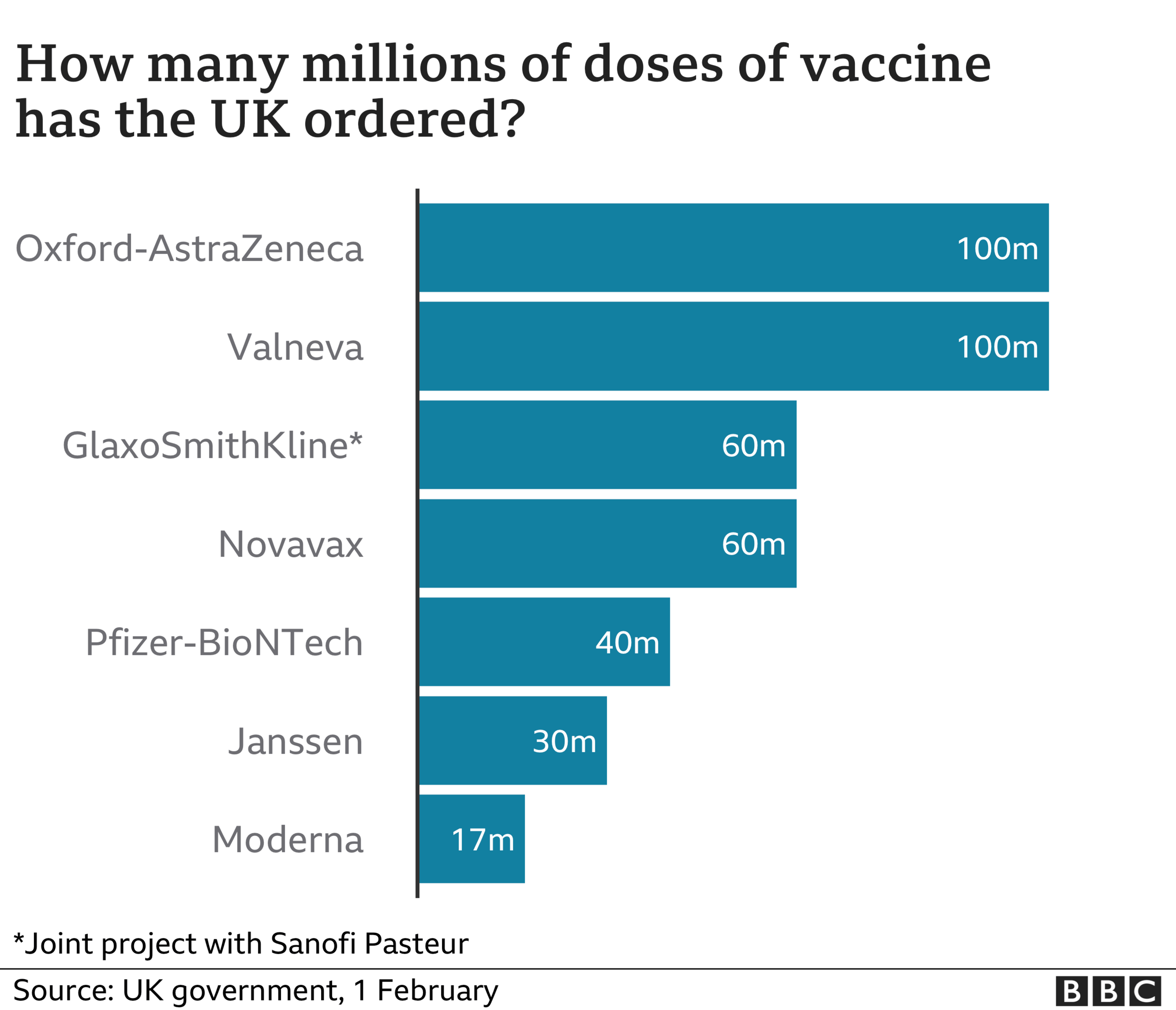
There are more than enough supplies of approved vaccines to give every adult in the UK both doses.
Data suggests that whilst the efficacy of both vaccines is optimised when a second dose is administered, they offer considerable protection after a single dose, at least in the short term.
So the new plan is for more people to be given a first dose, rather than holding back stock to administer down the line as second doses.
Everyone should still receive a second dose within 12 weeks of the first.
What's the difference between these vaccines?

Trials of the Oxford vaccine, which was developed by British drug manufacturer AstraZeneca and Oxford University, show it stops an average of 70% of people developing Covid symptoms.
Nobody who was given the vaccine in the trial developed severe disease or needed hospital treatment.
There is also data that suggests adjusting the dose could increase protection up to 90%.
This vaccine takes a weakened version of a common cold virus from chimpanzees and genetically modifies it to resemble coronavirus to try to provoke an immune response.
The manufacturers of the Pfizer/BioNtech vaccine say the jab could prevent 95% of people from becoming ill with Covid-19.
It is what's known as an RNA vaccine - a new type of technology. It involves injecting part of the virus' genetic code into the body to train the immune system.
Antibodies and T-cells are then made by the body to fight the coronavirus.
There were fears that the requirement for it to be kept at ultra-cold temperatures would pose problems in getting it to care homes or to remote communities.
However, it has since transpired that the vaccine can be moved around in an unfrozen state for up to 12 hours and can be stored undiluted for up to five days.
The Moderna vaccine is an RNA vaccine, like Pfizer's, which uses part of the virus's genetic code in order to provoke an immune response.
It requires temperatures of around -20C for shipping - similar to a normal freezer.
Several other vaccines are in the final testing stage, and more results from other teams working on advanced trials are also expected in the coming weeks and months.
Which one will I get?
The JCVI has said it does not have a preference for one vaccine above the other, but it's unlikely you'll get to choose which you get.
That depends on a number of factors - what is available, where you are being vaccinated, and what we know about how the vaccines work by the time you are being vaccinated.
The Scottish government has said that it will be distributing supplies so that they can offer the most "extensive and timely" coverage possible, so for that reason "one vaccine may be offered in certain settings over another".
And scientists will be studying the efficacy of the injections as people are given them, and it may well turn out that different vaccines suit different parts of the population.
So, it may be that what vaccine you get is dependent on your age or where you live, for example. But it is early days - we'll learn more in the months ahead.
Who can't be vaccinated?

Public health officials said when the Pfizer jab was approved that pregnant and breastfeeding women should not be given the injection, due to the limited amount of data available on its use within that group.
However, the regulator has since updated its advice to say that when the potential benefits outweigh the risks, the decision can be made for the woman to be vaccinated.
For the Oxford vaccine, the advice is the same - it can be administered when the potential benefits outweigh the risk.
Most children aged 18 and under will not get the Pfizer vaccine. This is because not enough research on using this vaccine on children has been carried out yet.
And, so far, the Oxford vaccine has only been approved for those over 18.
Regulators initially said that people with a history of significant allergic reactions should not have the Pfizer/BioNTech Covid jab.
But they have now said they have received further assurances, and that those with allergies to food or other medicines or vaccines can have this injection.
However, anyone with a history of allergic reaction to this vaccine or any of its ingredients should not receive this injection.
The Oxford vaccine can also be given to those with allergies to other medicines or food.
Again, it's only those with a known history of reacting to any of its ingredients who should not have it.
Anyone who has previously reacted to a vaccine should speak to their doctor first.


- Published30 December 2020

- Published7 May 2021
- Published28 May 2021