Families in NHS funding plea for life-changing cystic fibrosis drug
- Published

Fiona, Zara and Calum Stewart
Families of children diagnosed with cystic fibrosis say they are in a race against time to get their child prescribed a life-changing drug.
When Zara Stewart, two, was diagnosed with the condition at 18 days old, her parents were told that she would lead a normal life because of Kaftrio.
Kaftrio is a drug which can enhance the life expectancy of someone with cystic fibrosis by around forty years.
But now its availability on the NHS is under threat.
Zara's mother, Fiona, said: "Zara is on a number of medications at the moment, but these modulators are the first drugs to actually work with the cause of cystic fibrosis.
"The medications she's on help with the side-effects that she gets with cystic fibrosis, but modulators work with the cause of it."
A prescription for the drug, made by pharmaceutical company Vertex, can be up to £180,000 per year.
A consultation by the National Institute for Health and Care Excellence (NICE) recently investigated whether or not Kaftrio was too expensive to be available on the NHS.

Zara Stewart is now eligible to receive a life-extending drug
Although the final decision isn't expected until early next year, draft guidance issued in November stated that the drugs are too expensive.
Fiona, from Stepps, North Lanarkshire, continued: "I just think it's heart-breaking.
"We read something that said that they want to make sure that the taxpayer is getting value for money and to hear that said about your own child's life is horrible.
"I do appreciate it is a lot of money but these are drugs that are extending the quality of life for so many people, I don't understand why they wouldn't choose to pay for it."
Crucially, both NICE and the Scottish Medicines Consortium (SMC) have stated that those already prescribed Kaftrio, and those who get prescribed the drug between now and the enactment of any decision, will continue to get the drug for the rest of their lives.
With the age of prescription also recently dropping from six to two, many families of children aged between two and five are trying to get their child onto the drug before the final decision.

Stuart Milne, Jacob and his mother Morag
Stuart Milne's son Jacob is three and has been on a modulator for cystic fibrosis since he was born.
Living in Aberdeen, Jacob is waiting to be prescribed Kaftrio but now his parents are in limbo following the NICE consultation, which Stuart said "came out of the blue".
"It provides the person with a high quality of life and it's as close as we've come to a cure," said Stuart.
"As a parent of a young child born with this condition the message was always it's all different now because of the drugs we have.
"All of it is being taken away because Vertex is putting on too high a price."
For families with children under the age of two there is even more uncertainty as they are still too young be prescribed Kaftrio.
Marc Cotterill from Staffordshire got access to Kaftrio when he was 37.
At that stage, his lung capacity was at 30 per cent, one of his lungs had collapsed and he had been undergoing six hour treatments everyday to clear a build up of mucus in his lungs.
Now 41, Marc says the drug has changed his life.
He's gone from being unable to fly due to his health to taking up paddle boarding and running 5ks.
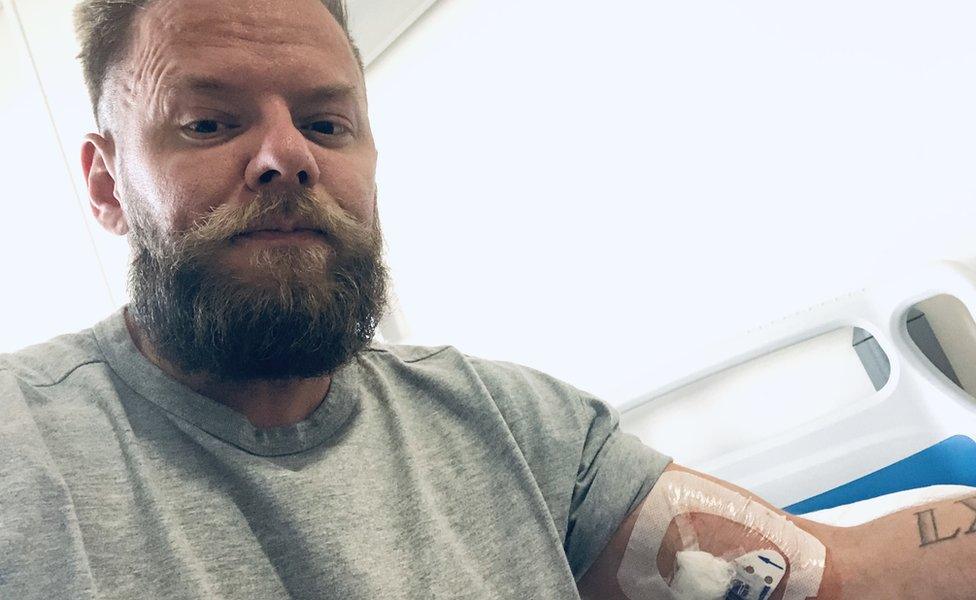
Marc Cotterill says the drug has changed his life
He told BBC Scotland News: "The important thing is to recognise for children who might gain access to this, and for some who have already gained access from a very young age, the important thing for them is they are likely to avoid all these complications that I have experienced.
"I think this is so important because while they won't experience the transformation like I did, thankfully because they've not had that time in order to decline, it's likely in many cases they'll avoid the horrible hospitalisations, IV antibiotics, treatments and all the things I've been through over my lifetime."
Vertex said that the list price published when the drugs were first authorised was not reflective of the price negotiated with local health boards like the NHS.
They were however unable to tell BBC Scotland News what the negotiated price was or if it was lower than the list price.
A spokesperson for the company said: "We have worked tirelessly for over 20 years to design, discover and develop CF medicines to treat the underlying cause of the disease and we continue to invest heavily in our research efforts.
"Each year, we reinvest over $1bn into developing new medicines for people with CF, and others, who today have few or no treatment options."
A spokesperson for the SMC said the draft recommendations could change following consultation and engagement.
Related topics
- Published26 November 2023

- Published24 November 2023

- Published24 November 2023
