Cystic fibrosis: New drug Kalydeco refused for Welsh NHS
- Published
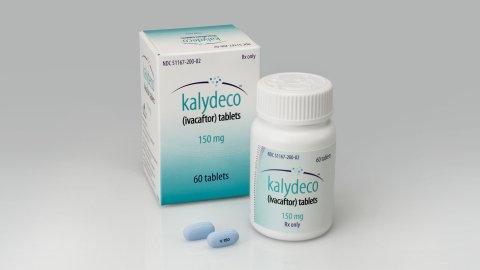
Kalydeco, also known as Ivacaftor, treats the root cause of cystic fibrosis for some sufferers
Drugs that could transform the lives of some cystic fibrosis sufferers should not be available on the Welsh NHS, Welsh government advisers say.
Kalydeco is authorised in England, Scotland and Northern Ireland but it has not been recommended in Wales because of its cost.
Health Minister Mark Drakeford will now take the final decision on the drug.
The Cystic Fibrosis Trust has warned that patients in Wales could be faced with a second class health service.
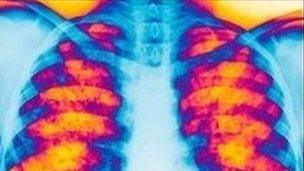
The life-threatening genetic disorder damages the lungs and digestive system.
Kalydeco is said to be the first drug to treat the root cause of cystic fibrosis for people with the G551D mutation and could dramatically improve the life expectancy of sufferers.
About 4% of patients across the UK have this mutation, equating to around 10 patients in Wales who could benefit, the Cystic Fibrosis Trust estimates.
The All Wales Medicines Strategy Group (AWMSG), which advises Mr Drakeford, announced on Wednesday that it would not approve the drug for use on the Welsh NHS.
At an estimated cost of up to £180,000 per patient per year, it said the drug, also known as Ivacaftor, was not cost-effective.
But the Cystic Fibrosis Trust said the medicine was "transformational", had a "dramatic effect" and that experts say "no other medicine addresses the basic genetic defect in cystic fibrosis".
It said it was "bitterly disappointed" by the decision but that the fight would not stop.
It is urging families and patients with cystic fibrosis, including those with hopes for future drugs in the pipeline, to contact their assembly members and ask them to lobby Mr Drakeford not to accept the recommendation.
Ed Owen, chief executive of the trust, said: "It would be an outrage if people in Wales with cystic fibrosis were prevented from receiving a treatment that is freely available on the health service in the rest of Britain.
"People in Wales should not be treated as second class citizens in the National Health Service."

Rose Maunder's mother hopes future drugs could help her cystic fibrosis
Patients and their families say the drug will also help the development of future medicines which could improve the lives of people with the more common mutation of cystic fibrosis - delta F508.
Judith Maunder, whose youngest daughter Rose, 22 months, has the delta F508 form of cystic fibrosis, said: "I'm concerned they have only looked at a small proportion of people who it will 100% help.
"But for people like my daughter - and there are about 70% in Wales who have the delta F508 mutation - Kalydeco is also important.
"There are currently advanced trials going on into drugs that could cure the delta F508 mutation. But the new trials work on the basis that Kalydeco is used in combination with the other drugs."
She said ever since Rose was diagnosed with cystic fibrosis when she was five weeks old she has dedicated her life to ensuring her daughter's lungs remain as healthy as possible in the hope that a cure is found within the next few years.
Mrs Maunder, of Dinas Powys in the Vale of Glamorgan, added: "While there are delays in getting these drugs authorised, there are little children in Wales whose lungs are being irrevocably damaged while we wait."
Bethan Jenkins, Plaid Cymru AM for South Wales West, said aside from the benefits that Kalydeco would bring patients, there could be wider implications in failing to authorise the drug.
"Ivacaftor is a 'first in class', and is expected to be followed by a series of similarly transformative drugs," she said.
"If we don't have this drug, patient trials are unlikely to follow, which would impact upon medical research and potentially further limit the numbers of doctors prepared to come and work in Wales."
A Welsh government spokesperson said: "Following the appraisal of any new medicine, the AWMSG forward their advice to the minister and all relevant issues are considered carefully before a final decision is taken.
"Ivacaftor/Kalydeco will be subject to the same process."
- Published20 January 2013
- Published17 January 2013

- Published14 January 2013
- Published3 October 2012
