Covid vaccine's impact on older people unclear, says first minister
- Published
Covid: 'A long way to go until everyone is protected'
It is not yet known how effective a new vaccine for coronavirus will be for older people, Wales' first minister has said.
Mark Drakeford appealed for caution after early results showed a vaccine by Pfizer and BioNTech could prevent 90% of people from getting Covid.
He said he did not want people in Wales to take the wrong message.
The Welsh Government is planning to deploy the vaccine should it win regulatory approval for use.
The first minister has said the UK government would buy the vaccine on behalf of the whole of the UK, with Wales getting a share reflecting its share of the population.
Adam Price, Plaid Cymru leader, said the first minister "should commit to obtaining a share of the vaccine based on need rather than population".
Should the vaccine pass safety tests, the Welsh NHS will begin to immunise in December, with different groups to be prioritised.
"Health and social care workers, care home residents and staff have been prioritised to receive a vaccine first, with roll-out to older people in age bands from next year," a Welsh Government spokesman said.
"There will be limited supplies of a vaccine at first, so it will be offered to those at highest risk."
Mr Drakeford told members of the Senedd there were still unanswered questions about the Pfizer vaccine, including "whether or not it will be effective with older people".
He said care home residents and people in "later stages of life" would get the next priority after health and social care staff, if it is effective.
Concern at 'triumphalist' tone
The first minister accused "some of the right-wing press" of reporting in a "triumphalist way" Pfizer and BioNTech's announcement.

Mark Drakeford agreed the vaccine offered a "glimmer" of hope
Speaking in the Senedd, he said: "There are very important steps that have to be taken before that vaccine, or any of the other 11 vaccines that are at Stage 3 trials, might finally come to fruition.
"I really, really do not want people in Wales to take the wrong message from what was being said yesterday.
"We will be fighting coronavirus with the current armoury that we have at our disposal for many months to come and, while we look forward to the day when there is a vaccine, we need to be cautious in the way we approach it."
But Mr Drakeford agreed with a comment from Adam Price that the vaccine offered a "glimmer" of hope.
"It is hopeful and we need to have hope in the these difficult times, but we mustn't exaggerate it either," the first minister said.
He added that storage and distribution will fall under the Welsh Government's responsibility, which was "particularly important" for the Pfizer vaccine because it "can only properly be stored at very low temperatures".
Facilities from the Welsh Blood Service would be used for the vaccine, Mr Drakeford said, which had "real constraints" - it can only be taken out of refrigeration on four occasions, and has to be administered twice at a two to three-week interval.
The first minister said there may later be a "case for additional volumes of vaccine coming to Wales and to make sure that the prioritisation that we agree is fine-tuned to meet the needs of Wales' population".
Commit to 'share of vaccine'
Mr Price, Plaid leader, said: "The first minister should commit to obtaining a share of the vaccine based on need rather than population, and he needs to provide clarity on who will be prioritised.
"Wales has some of the highest areas of infection in the UK and if - as I have called for today - the vaccine is prioritised for communities at highest risk of infection, Wales will need a greater share. "We do not want to find ourselves in the situation where the rest of the UK can return to normal, yet Wales hasn't had a sufficient amount of the vaccine to protect all of our most vulnerable."
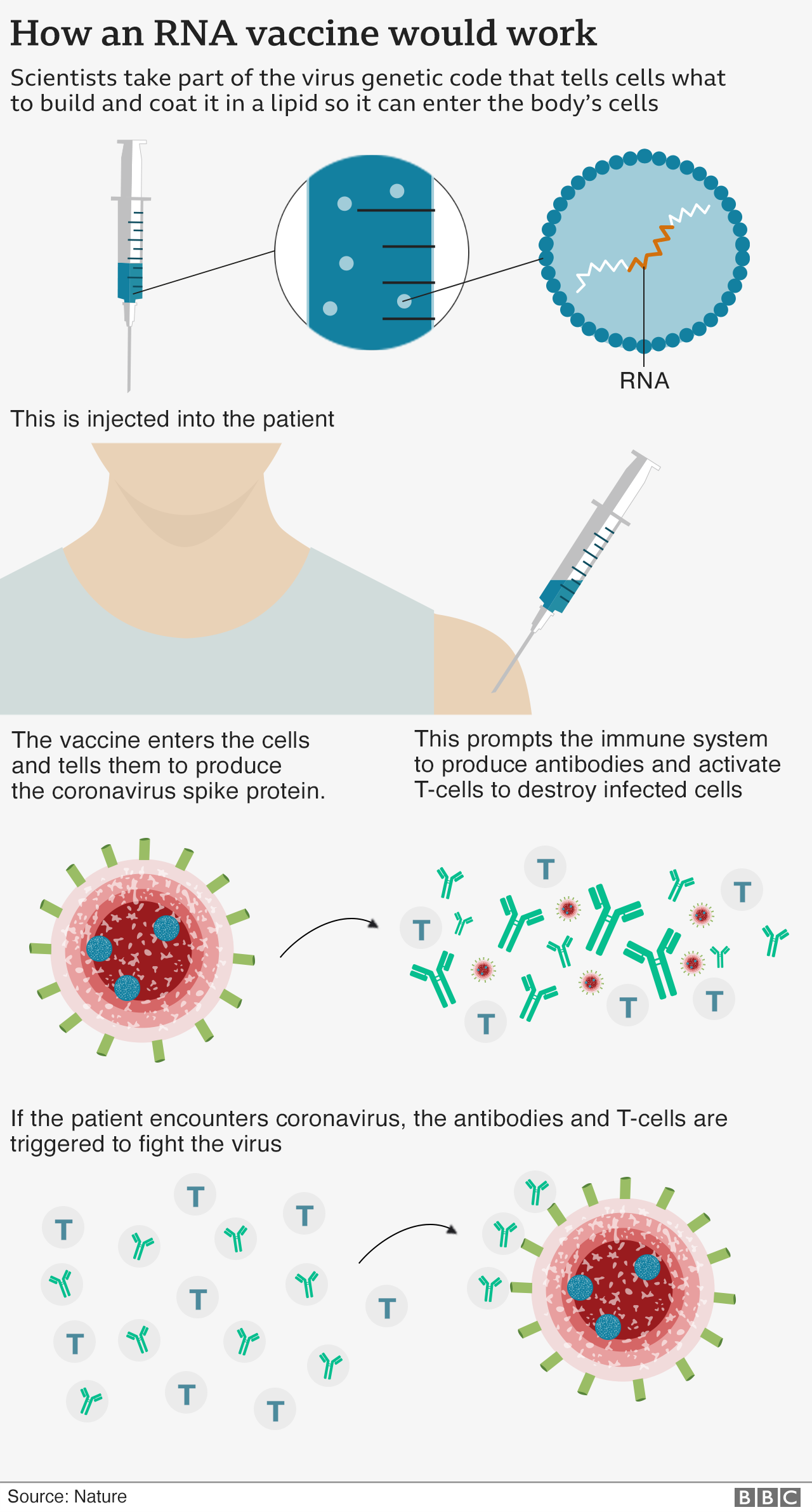

How would the vaccine work?
The vaccine - called an RNA vaccine - has been developed by pharmaceutical companies Pfizer and BioNTech and is one of 11 vaccines that are currently in the final stages of testing.
The companies now plan to apply for emergency approval to use the vaccine by the end of November - and a limited number of people may get the vaccine this year.
Until it has been approved it will not be possible for countries to begin their vaccination campaigns.
It has been tested on 43,500 people in six countries with no safety concerns raised.
The UK has already ordered 40 million doses - enough to vaccinate up to 20 million people as each person will need two doses for it to work effectively.
UK Health Secretary Matt Hancock has said the NHS is ready to start providing the new coronavirus vaccine "as fast as safely possible".
- Published9 November 2020