XLH: Family distraught as bone disease drug not approved
- Published

Claire Lewis says the "game-changer" drug had given hope to so many families
A family say they are devastated after medication described as a "cure" for their rare bone disease was not recommended for the NHS.
Claire Lewis, 59, and her family live with X-linked hypophosphataemia (XLH), which affects bones and teeth.
The National Institute for Health and Care Excellence (NICE) rejected the burosumab drug for adults on the NHS in draft guidance, external.
"It affects every part of your life… we are distraught," said Ms Lewis.
Ms Lewis, from Hirwaun, Rhondda Cynon Taf, said the new medication, marketed under the name Crysvita, could be a "game-changer".
"I've been told it's [as good as] a cure," she said.
Approval by the Scottish Medicines Consortium means patients in Scotland have been able to access the drug, external on the NHS since February.
Four generations of Ms Lewis' family - including her three siblings, her mum, her three children and her great-nephew Reggie - have the hereditary condition and they can trace it back to the 1800s in their lineage.
They face daily pain, and one of her sisters has even lost all her teeth to the condition.
What is XLH?
XLH affects one in 20,000 people and Ms Lewis believes only eight families in Wales are affected.
But "there are absolutely no expertise for adults with XLH" in Wales, she added.
It is characterised by low levels of phosphate and "basically gives you rickets".
Ms Lewis was a teacher for more than 20 years, but had to give up her career a decade ago when she needed surgery.
"It affects every single part of your life," she said.
"As I've got older, the fatigue has completely taken over. I can only do half of what I used to, 59 is not old."
She said even getting up and getting dressed were difficult.
"I can get up in the morning feeling as if I haven't slept at all, even though I may have slept 12 hours," she added.
"We are very weak because we can't walk that far. I can no longer get on and off buses or trains."

Claire Lewis, right, and her daughters all suffer daily pain due to the bone disease
Ms Lewis said her marriage broke down due to the stress, and that relationships were often difficult for people with XLH, partly due to the possibility of passing the disease to children.
She added: "What it affects most is your mental health… being different and people just not understanding what's happening."
Ms Lewis' family currently attend a geriatric bone clinic, since the funding for them to go to a specialised clinic in Oxford was stopped in 2018.
"The treatment in Wales is, I would like to say poor, but I have to say non-existent," she said.
Stuart Ralston, a professor of rheumatology at the University of Edinburgh, is an expert in bone diseases and has used burosumab with some of his own patients since February.
"It's worked fantastically well. Essentially, it's like a cure, so it's a very effective treatment," he said.
Prof Ralston said NICE "seem to be stalling" due to less evidence available on the effects of the medication in adults compared to children and hoped its use in Scotland would help demonstrate the benefits.
People with XLH have a lack of active vitamin D in their bodies, which needs to be activated before the body can use it "a bit like your electronic train tickets on your mobile phone," said Prof Ralston.
He added phosphate supplements were "hard for patients to take" making it difficult to control the condition without burosumab.
He said he did not know how much the NHS would have to pay for it, but admitted it was "very expensive" at a list price of about £200,000 a year per patient.
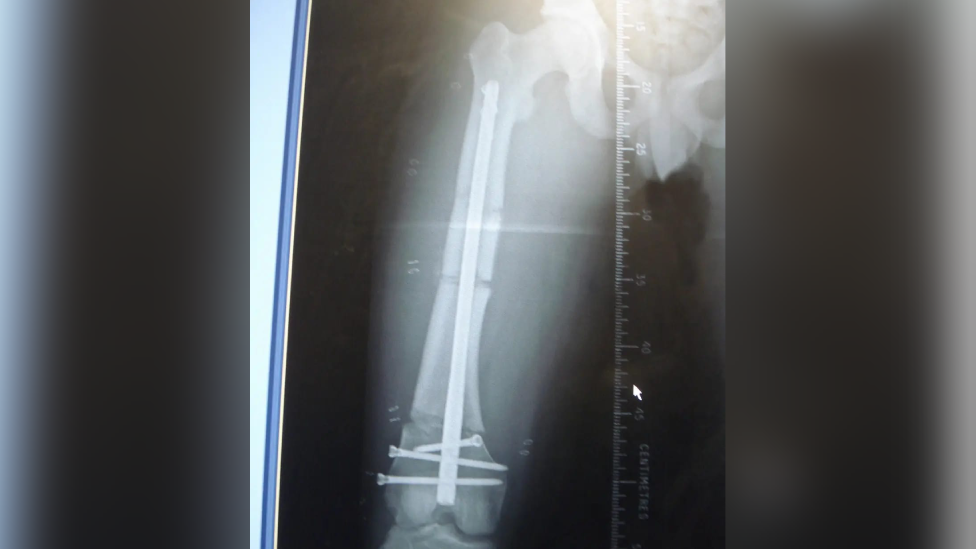
Claire Lewis had to have surgery to her femur, forcing her to give up work when she was just 48 years old
Ms Lewis attended a roundtable discussion on rare diseases at the Senedd, chaired by Mike Hedges, on 4 October.
Swansea East MS Mr Hedges said he was "disappointed" by the NICE decision, but added: "I would like to see an arbitration procedure on the price of drugs so that companies would need to justify the price they are charging."
In its draft guidance, NICE said "people having burosumab may have less pain and fatigue, and improved physical functioning" but this was "not certain".
It added: "All of the cost effectiveness estimates are above the range normally considered an acceptable use of NHS resources."
The treatment has previously been approved by NICE for children with growing bones, external, but Ms Lewis said this was concerning because young people like three-year-old Reggie would not have an understanding of the condition's symptoms when the treatment stopped.
"The difference in him has been outstanding," Ms Lewis said.
She added the XLH community - who communicate online and regularly meet up - have been waiting years to find out if the drug would be available to them.
"Everyone is devastated... It's so short-sighted," Ms Lewis said.

Reggie, three, is receiving the burosumab drug but his family are worried about how he will cope when he turns 18 and funding stops
The second NICE evaluation is in February 2024 and campaigners are lobbying NICE to reconsider.
Charity XLH UK said: "Access to this treatment is not merely about managing symptoms, it's about providing individuals the opportunity for a life without the constant burden of pain and limited mobility."
It said denying access to the drug "imposes a cycle of unnecessary additional suffering."

Claire Lewis and other XLH patients and campaigners attended a round table discussion at the Senedd in October
Kyowa Kirin, the company which makes the drug, said it "regrets" NICE's decision, but is committed to finding a solution.
The Welsh government said it relied on NICE guidance "to ensure resources are targeted where patients will benefit the most, and where the medicine costs are in balance with those benefits".
"The draft guidance does not constitute NICE's final position on the availability of burosumab," it said.
Related topics
- Published23 February 2023
- Published25 October 2022

- Published16 September 2023
