Volunteering to test Ebola vaccine in Mali
- Published
Ebola vaccine trial expands in Mali
Preliminary indications in Mali from the first trial for an Ebola vaccine taking place in Africa seem promising.
"We know that after two weeks they're starting to have some immune response and there are no adverse reactions," says Dr Samba Sow, an infectious disease epidemiologist and vaccine expert and director of Centre for Vaccine Development (CVD) in Mali.
The first volunteer in Mali was paediatrician Dr Seydou Sissoko, who was vaccinated on 8 October at the CVD in the capital, Bamako.
"Since I received the vaccine, I feel well, there is no difference in the way a feel now and how I felt before," he said.
Ten other healthcare workers are due to receive more Ebola vaccines on Thursday in the trial.
The exercise is part of a bigger multi-country study which began in the UK in September.
It is a joint effort involving the World Health Organization (WHO), the US National Institutes of Health and UK pharmaceutical firm GlaxoSmithKline.
It is also one of the most fast-tracked vaccines in history - with experts hoping to find a way to develop immunity to Ebola - which has killed nearly 5,000 people this year in West Africa - in a matter of months.
"What motivated me to join the study was the fact that I am a medical doctor and I'll be regularly in touch with patients of Ebola," says Dr Sissoko.
This week he has been asked to investigate two suspected cases of Ebola - one of them was a man who died after bleeding from the mouth.

People crossing the border into Mali from Guinea are having their temperatures checked
"It is first to protect myself and secondly to help the scientific community find a good vaccine against the disease."
At first, his family were worried about his participation in the trial, which is being funded from the research charity the Wellcome Trust, the UK's Medical Research Council and Department for International Development.
"I explained to my wife and brother how it works and now they are fine with it.
"But I have not talked about it to my parents because I thought they may not understand," he adds.
Double dosage
In total 40 volunteers are taking part in the Mali study - all of them are health workers who could be called upon to deal with Ebola should more cases appear in the country.
The first and only case has been that of a two-year-old girl who travelled from one of the worst-affected countries, Guinea, in October and later died of the disease.
Dr Sow, Mali's newly appointed Ebola tsar, is also taking part in the trial at the CVD.
"It's a very smart vaccine that is very well known, so we hope that if the vaccine works we'll be protected and we'll not be too scared to do our job in the frontline," he told the BBC as he was vaccinated on Tuesday along with a nine other volunteers.
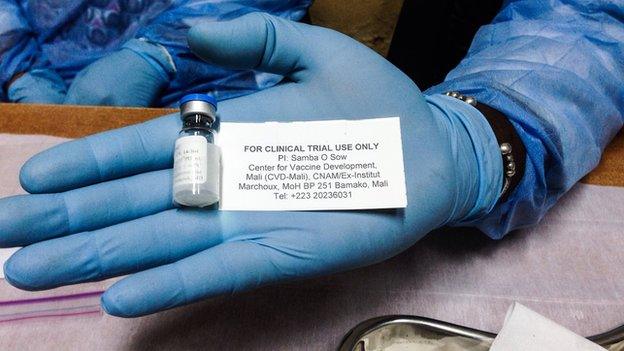
Doses of the vaccine are removed from the freezer and left to thaw in room temperature
One after the other they walked into the vaccination room and sat facing two colleagues who were clad in blue protective coats, goggles and gloves.
"This is a very special occasion where we have to let study investigators be participants," he said.
This lot of participants received double the dosage given to earlier volunteers.
The idea is to not only investigate if the drug ignites an immune response, but also find out what dosage would be effective.
The vaccines are kept in temperatures of approximately minus 80C in an adjacent room, which only two members of staff have access to - and the refrigerator is kept locked.
About 20 minutes before the next participant arrives, a dose of the vaccine is removed from the freezer and left to thaw in room temperature.
The current outbreak is the deadliest since Ebola was discovered in 1976
It is then carefully extracted into a syringe.
Dr Sow explains that unlike many other vaccines where a weakened form of the live virus is used, in this study a modified chimpanzee common cold virus is used to carry a single Ebola protein.
It cannot infect someone with Ebola, but should prompt the production of antibodies against the virus.
"The chimp virus can only give a cold but not get somebody really sick," he assures.
It is a tried-and-tested vaccine carrier, he says.
For this phase of the trial, none of the participants will receive a placebo.
The volunteers are observed for the first hour after vaccination.
They give blood samples before being inoculated and then a day, a week, two weeks and 28 days afterwards.
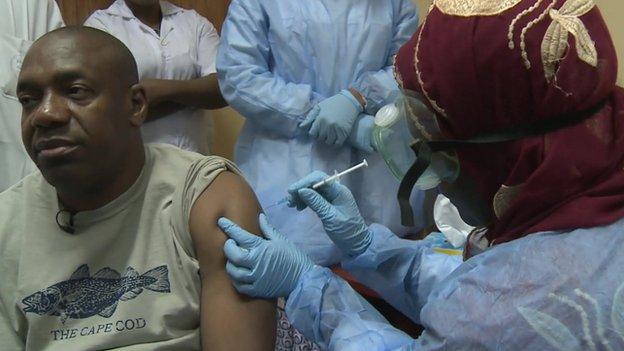
Dr Sow says he hopes a new vaccine would stop an epidemic becoming a pandemic
The samples are processed in a laboratory at the research centre.
The scientists extract white blood cells - the part of the blood that fights diseases - and observe them under a microscope to see if there is an immune response.
But they have to send them to the University of Maryland in Baltimore, US, for more sophisticated analysis to determine if the immunity - if any - is against Ebola.
"What we're hoping is to have this vaccine as quickly as possible so it can help us to better control this epidemic and also to help us not to have this epidemic become a pandemic," says Dr Sow.
At home with his wife and son, Dr Sissoko says a new vaccine would give his family some reassurance as they constantly worry about his chance of contracting the virus given the risk health workers face, with more than 300 dying so far in the current outbreak.